Fulvestrant
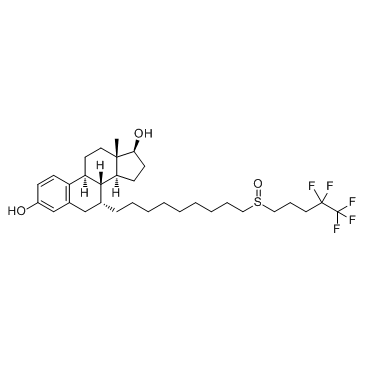
Fulvestrant structure
|
Common Name | Fulvestrant | ||
|---|---|---|---|---|
| CAS Number | 129453-61-8 | Molecular Weight | 606.771 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 674.8±55.0 °C at 760 mmHg | |
| Molecular Formula | C32H47F5O3S | Melting Point | 104-106°C | |
| MSDS | Chinese USA | Flash Point | 361.9±31.5 °C | |
Use of FulvestrantFulvestrant is a potent Estrogen Receptor antagonist with an IC50 of 9.4 nM. |
| Name | fulvestrant |
|---|---|
| Synonym | More Synonyms |
| Description | Fulvestrant is a potent Estrogen Receptor antagonist with an IC50 of 9.4 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 9.4 nM (Estrogen Receptor)[1] |
| In Vitro | Fulvestrant (ICI 182,780) is a potent and specific inhibitor of estrogen action and demonstrates excellent growth-inhibitory effects in both cell and animal models of human breast cancer. Fulvestrant inhibits MCF-7 human breast cancer cells growth with IC50 of 290 nM. The relative binding affinities of Fulvestrant is 0.89 compare with that of Estradiol. Fulvestrant has significantly increased antiestrogenic potency and retains pure estrogen antagonist activity[1]. Fulvestrant is the first of a new type of endocrine treatment-an oestrogen receptor (ER) antagonist that downregulates the ER[2]. Treatment of MCF-7 cells with 1 µM Tamoxifen has no effect on the expression of ERα, whereas 100 nM Fulvestrant completely inhibits ERα expression[3]. |
| In Vivo | When administered alone, parenterally (s.c.), to immature female rats Fulvestrant (ICI 182,780) is devoid of uterotropic activity and, when coadministered with Estradiol, it effectively blocks the uterotropic action of Estradiol in a dose-dependent manner (ED50: 0.06 mg/kg/day s.c.). Complete antagonism of Estrogen action is achieved with a dose of 0.5 mg Fulvestrant/kg/day s.c. The effects of Fulvestrant administered p.o. are qualitatively similar but potency is reduced by an order of magnitude compare with s.c. dosing (ED50 0.46 and complete antagonism at 5 mg/kg/day p.o.)[1]. The antitumour activity of Fulvestrant is first demonstrated in two models of human breast cancer in nude mice. In one of these models, the growth of MCF-7 tumour xenografts, supported by continuous treatment with oestradiol, is completely blocked for at least 4 weeks following a single injection of Fulvestrant 5 mg. Similar reductions in growth are seen in the Br10 human tumour model. In other studies in nude mice bearing MCF-7 xenografts, Fulvestrant suppresses the growth of established tumours for twice as long and tumour growth is delayed to a greater extent than is observed with Tamoxifen treatment. Tamoxifen-resistant breast tumours, which grow in nude mice after long-term treatment with Tamoxifen, remain ensitive to growth inhibition by Fulvestrant[2]. These are comparable to the tumor growth inhibition (TGI) observed for Tamoxifen and Fulvestrant, which on day 40 are 86 and 88%, respectively[3]. |
| Cell Assay | MCF-7 or T47D cells are cultured in 10 cm dishes to ~75% confluence in EMEM growth medium supplemented with 10% FBS and 10 µg/mL human insulin. Twenty-four hours before treatment, the growth medium is replaced with phenol red-free RPMI-1640 growth medium. A stock solution of 10 mM RAD1901 is prepared in DMSO. Dilutions of RAD1901 are prepared in RPMI growth medium (doses ranging from 10 to 0.5 nM). Controls include 0.1% DMSO alone (vehicle), 1 nM Estradiol (E2), 100 nM Fulvestrant, and 1 µM Tamoxifen. Plated cells are treated with RAD1901 or controls for 48 h, and then incubated for 15 min with ice-cold lysis buffer [1 mM EDTA, 0.5% Triton X-100, 5 mM NaF, 6 M urea, 1 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, and 1× HALT protease inhibitor cocktail]. Lysates are centrifuged at 2000g for 5 min, and the supernatant is diluted 1 : 1 in lysis buffer. Ninety-six-well plates are coated overnight with capture antibody (1 µg/mL), washed three times in the manufacturer’s wash buffer, blocked with blocking buffer for 2 h, and washed again. The prepared plates are incubated with 100 µL of the prepared cell lysate for 2 h, washed, incubated with biotinylated detection antibody for 2 h, and washed again. After a 20 min incubation with streptavidin-horseradish peroxidase, the plates are washed and incubated with substrate solution for 20 min. The reaction is stopped with stop solution, and the plates are analyzed on a microplate reader (OD450)[3]. |
| Animal Admin | Rats[1] In studies with OVX rats, surgical preparation is performed at least 2 weeks before treatment began. To measure the duration of action of a single large dose of Fulvestrant, OVX rats are treated with a daily s.c. dose of 0.5 μg of estradici benzoate beginning on the day of Fulvestrant administration and continued until vaginal smears showed evidence of cornification. At that point the experiment is terminated and uterine weight is recorded. The arachis oil formulation used in these single dose duration of action studies contained 50 mg Fulvestrant/mL. Mice[3] Female athymic nude mice [Crl:NU(NCr)-Foxn1nu] are used for tumor xenograft studies. Fourteen days after tumor cell implantation (designated as day 1 of the study), mice are 9 weeks of age, with body weights ranging from 21.4 to 32.5 g, individual tumor volumes ranging from 75 to 144 mm3, and a group mean tumor volume (MTV) of 108 mm3. The mice are randomized into nine groups of 15 animals each and treated with vehicle, Tamoxifen (1 mg/animal every other day), Fulvestrant (0.5 mg/animal daily), or RAD1901 (0.3, 1, 3, 10, 30, 60, 90, and 120 mg/kg daily). Tumor volumes are evaluated twice per week. The tumor endpoint is defined as an MTV of 1500 mm3 in the control group. Animals are also monitored for partial regression (PR) and complete regression responses. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 674.8±55.0 °C at 760 mmHg |
| Melting Point | 104-106°C |
| Molecular Formula | C32H47F5O3S |
| Molecular Weight | 606.771 |
| Flash Point | 361.9±31.5 °C |
| Exact Mass | 606.316589 |
| PSA | 76.74000 |
| LogP | 7.92 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.522 |
| InChIKey | VWUXBMIQPBEWFH-WCCTWKNTSA-N |
| SMILES | CC12CCC3c4ccc(O)cc4CC(CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)C3C1CCC2O |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| RTECS | KG7623000 |
| HS Code | 2930909090 |
|
~86% 
Fulvestrant CAS#:129453-61-8 |
| Literature: XI'AN LIBAND PHARMACEUTICAL CO., LTD. Patent: US2010/174101 A1, 2010 ; Location in patent: Page/Page column 4 ; |
|
~31% 
Fulvestrant CAS#:129453-61-8 |
| Literature: SYNTHON B.V.; ETTEMA, Gerrit Jan Bouke; GIELING, Reinerus Gerardus Patent: WO2010/43404 A1, 2010 ; Location in patent: Page/Page column 32 ; |
|
~% 
Fulvestrant CAS#:129453-61-8 |
| Literature: US2006/30552 A1, ; Page/Page column 26 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Combined histone deacetylase inhibition and tamoxifen induces apoptosis in tamoxifen-resistant breast cancer models, by reversing Bcl-2 overexpression.
Breast Cancer Res. 17 , 26, (2015) The emergence of hormone therapy resistance, despite continued expression of the estrogen receptor (ER), is a major challenge to curing breast cancer. Recent clinical studies suggest that epigenetic m... |
|
|
PKA signaling drives mammary tumorigenesis through Src.
Oncogene 34(9) , 1160-73, (2015) Protein kinase A (PKA) hyperactivation causes hereditary endocrine neoplasias; however, its role in sporadic epithelial cancers is unknown. Here, we show that heightened PKA activity in the mammary ep... |
|
|
Elevated peritoneal expression and estrogen regulation of nociceptive ion channels in endometriosis.
J. Clin. Endocrinol. Metab. 99(9) , E1738-43, (2014) Ovarian suppression is a common treatment for endometriosis-associated pelvic pain. Its exact mechanism of action is poorly understood, although it is assumed to reflect reduced production/action of e... |
| (7R,8R,9S,13S,14S,17S)-13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol |
| MFCD00903953 |
| (17β)-7-{9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl}estra-1,3,5(10)-triene-3,17-diol |
| Estra-1,3,5(10)-triene-3,17-diol, 7-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]-, (17β)- |
| Fulvestrant |
![(7a,17b)-7-[9-[(4,4,5,5,5-Pentafluoropentyl)thio]nonyl]-estra-1,3,5(10)-triene-3,17-diol structure](https://image.chemsrc.com/caspic/409/153004-31-0.png)
![(7R,13S)-3-methoxy-13-methyl-7-(9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl)-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-ol structure](https://image.chemsrc.com/caspic/018/1221256-46-7.png)
![(7a,17b)-7-7-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]-estra-1,3,5(10)-triene-3,17-diol 17-acetate structure](https://image.chemsrc.com/caspic/157/261506-24-5.png)

