[29H,31H-Phthalocyaninato(2-)-κ2N29,N31]iron
![[29H,31H-Phthalocyaninato(2-)-κ2N29,N31]iron Structure](https://image.chemsrc.com/caspic/062/132-16-1.png)
[29H,31H-Phthalocyaninato(2-)-κ2N29,N31]iron structure
|
Common Name | [29H,31H-Phthalocyaninato(2-)-κ2N29,N31]iron | ||
|---|---|---|---|---|
| CAS Number | 132-16-1 | Molecular Weight | 568.368 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C32H16FeN8 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Name | Iron phthalocyanine |
|---|---|
| Synonym | More Synonyms |
| Molecular Formula | C32H16FeN8 |
|---|---|
| Molecular Weight | 568.368 |
| Exact Mass | 568.084717 |
| PSA | 84.02000 |
| LogP | 1.46190 |
| Water Solubility | It is insoluble in water. It is soluble in conc. sulfuric acid. |
| Precursor 10 | |
|---|---|
| DownStream 4 | |
|
An efficient mimic of cytochrome P-450 from a zeolite-encaged iron complex in a polymer membrane.
Nature 370(6490) , 541-4, (1994) Many attempts have been made to mimic the catalytic oxidative properties of the enzyme cytochrome P-450. For homogeneous systems the mechanisms of oxidation can be readily determined but proper mimicr... |
|
|
Detection of pesticides using an amperometric biosensor based on ferophthalocyanine chemically modified carbon paste electrode and immobilized bienzymatic system.
Biosens. Bioelectron. 18(2-3) , 303-10, (2003) A new highly sensitive amperometric method for the detection of organophosphorus compounds has been developed. The method is based on a ferophthalocyanine chemically modified carbon paste electrode co... |
|
|
Bonding of nitrosobenzene and phenyl isocyanide to chelated mesoheme, hemoglobin and ferrous phthalocyanine.
Biochim. Biophys. Acta 955(2) , 220-30, (1988) Reactions of nitrosobenzene, phenyl isocyanide and their ring-substituted analogues with hemoglobin, ferrous phthalocyanine and a synthetic model compound of hemoglobin were investigated by optical, 1... |
| EINECS 205-047-2 |
| Ferrous phthalocyanine |
| MFCD00015953 |
| Iron(II) Phthalocyanine |
| Fe pythalocyanine |
| Iron, [29H,31H-phthalocyaninato(2-)-κN,κN]- |
| Phthalocyanine Iron(II) |
| [29H,31H-Phthalocyaninato(2-)-κN,N]iron |
| Phthalocyanine iron(II) salt |
| Fe phthalocyanine |
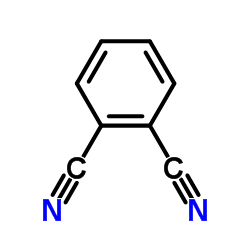 CAS#:91-15-6
CAS#:91-15-6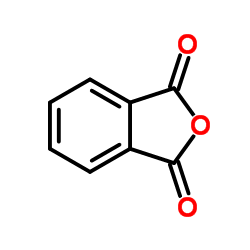 CAS#:85-44-9
CAS#:85-44-9 CAS#:7439-89-6
CAS#:7439-89-6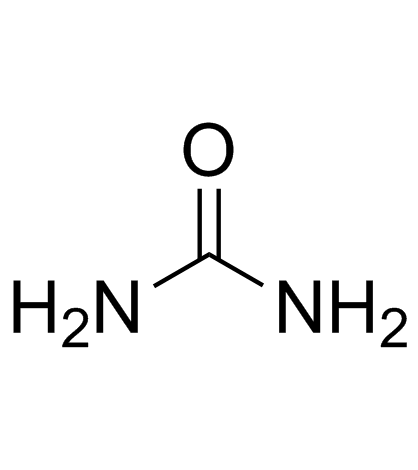 CAS#:57-13-6
CAS#:57-13-6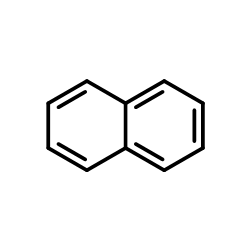 CAS#:91-20-3
CAS#:91-20-3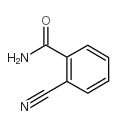 CAS#:17174-98-0
CAS#:17174-98-0 CAS#:102-54-5
CAS#:102-54-5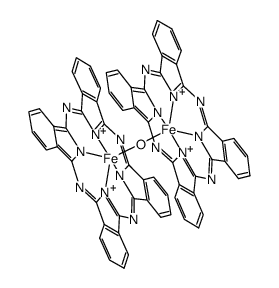 CAS#:74353-48-3
CAS#:74353-48-3 CAS#:74325-91-0
CAS#:74325-91-0 CAS#:13463-40-6
CAS#:13463-40-6 CAS#:7440-44-0
CAS#:7440-44-0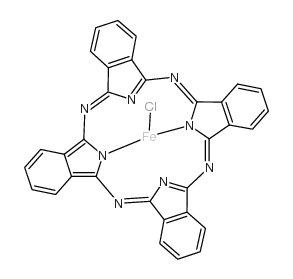 CAS#:14285-56-4
CAS#:14285-56-4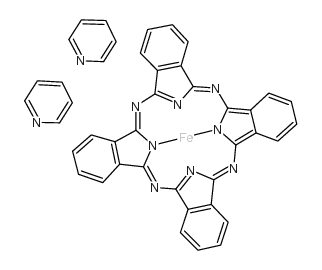 CAS#:20219-84-5
CAS#:20219-84-5