cerium(iii) oxalate
Modify Date: 2025-08-25 05:10:41
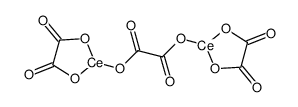
cerium(iii) oxalate structure
|
Common Name | cerium(iii) oxalate | ||
|---|---|---|---|---|
| CAS Number | 13266-83-6 | Molecular Weight | 544.28900 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6Ce2O12 | Melting Point | decomposes [STR93] | |
| MSDS | N/A | Flash Point | N/A | |
| Name | cerium(iii) oxalate |
|---|---|
| Synonym | More Synonyms |
| Melting Point | decomposes [STR93] |
|---|---|
| Molecular Formula | C6Ce2O12 |
| Molecular Weight | 544.28900 |
| Exact Mass | 543.75000 |
| PSA | 157.80000 |
| Appearance of Characters | crystal | white |
| Water Solubility | insoluble H2O; soluble H2SO4, HCl; insoluble oxalic acid, alkali, ether, alcohol [KIR78] |
|
Section 1: Product Identification Chemical Name:Cerium (III) oxalate nonahydrate (99.9%-Ce) (REO) CAS Registry Number:13266-83-6 Formula:Ce2(C2O4)3.9H2O EINECS Number:none Chemical Family:salt of an organic acid Synonym:cerium oxalate, Oxalic acid, cerium salt.
Section 2: Composition and Information on Ingredients IngredientCAS NumberPercentACGIH (TWA)OSHA (PEL) Title Compound13266-83-6100%no datano data Section 3: Hazards Identification Soluble oxalates are harmful if swallowed. They precipitate calcium from the bloodstream causing violent Emergency Overview: muscular stimulation, convulsions, collapse, and death. Primary Routes of Exposure:Ingestion Eye Contact:May cause moderate to severe irritation of the eyes. Skin Contact:May cause moderate to severe irritation of the skin. Inhalation:(If dust) May be severely irritating to the nose, mucous membranes and respiratory tract. Ingestion:Ingestion may cause vomiting, pain, violent muscular stimulation, convulsions, collapse, and death. Soluble oxalates remove calcium from the bloodstream. This interferes with the central nervous system Acute Health Affects: causing violent convulsions and death. Prolonged exposure to oxalates can lead to kidney failure resulting from calcium oxalate precipitation in renal Chronic Health Affects: tubes NTP:No IARC:No OSHA:No SECTION 4: First Aid Measures Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need Eye Exposure: assistance in keeping their eye lids open. Get immediate medical attention. Wash the affected area with soap and water. Remove contaminated clothes if necessary. Seek medical Skin Exposure: assistance if irritation persists. Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty Inhalation: in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance. Antidotes are said to be soluble calcium given orally, and calcium gluconate given intravenously, to be Ingestion: administered by trained medical personnel. Keep the victim calm. Give the victim water (only if conscious). SECTION 5: Fire Fighting Measures Flash Point:not applicable Autoignition Temperature:none Explosion Limits:none Extinguishing Medium:carbon dioxide, dry powder or foam If involved in a fire, fire fighters should be equipped with a NIOSH approved positive pressure self-contained Special Fire Fighting Procedures: breathing apparatus and full protective clothing. Hazardous Combustion andIf involved in a fire this material may emit toxic organic fumes. Decomposion Products: Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards. SECTION 6: Accidental Release Measures Spill and Leak Procedures:Small spills can be mixed with vermiculite or sodium carbonate and swept up. SECTION 7: Handling and Storage Handling and Storage:Store in a sealed container. Keep away from heat and moisture. SECTION 8: Exposure Controls and Personal Protection Eye Protection:Always wear approved safety glasses when handling a chemical substance in the laboratory. Skin Protection:Wear appropriate chemical resistant gloves and protective clothing. Ventilation:Material may form a fine dust. If possible, handle the material in an efficient fume hood. If in form of fine dust and ventilation is not available a respirator should be worn. The use of respirators Respirator: requires a Respirator Protection Program to be in compliance with 29 CFR 1910.134. Ventilation:Material may form a fine dust. If possible, handle the material in an efficient fume hood. Additional Protection:No additional protection required. SECTION 9: Physical and Chemical Properties Color and Form:white xtl. Molecular Weight:544.30 (706.44) Melting Point:dec. Boiling Point:no data Vapor Pressure:not applicable Specific Gravity:no data Odor:none Solubility in Water:slightly soluble SECTION 10: Stability and Reactivity Stability:air and moisture stable solid Hazardous Polymerization:no hazardous polymerization Conditions to Avoid:none Incompatibility:Oxidizing agents Decomposition Products:Carbon dioxide, carbon monoxide, organic vapors, and metal oxides and carbonates. SECTION 11: Toxicological Information RTECS Data:No specific information available on this product. Carcinogenic Effects:No data available Mutagenic Effects:No data available Tetratogenic Effects:No data available SECTION 12: Ecological Information Ecological Information:No information available SECTION 13: Disposal Considerations Disposal:Dispose of according to local, state and federal regulations. SECTION 14: Transportation Shipping Name (CFR):Non-hazardous Hazard Class (CFR):NA Additional Hazard Class (CFR):NA Packaging Group (CFR):NA UN ID Number (CFR):NA Shipping Name (IATA):Non-hazardous Hazard Class (IATA):NA Additional Hazard Class (IATA):NA Packaging Group (IATA):NA UN ID Number (IATA):NA SECTION 15: Regulatory Information TSCA:Not listed in the TSCA inventory. SARA (Title 313):Title compound not listed. Second Ingredient:none SECTION 16 - ADDITIONAL INFORMATION N/A |
| Hazard Codes | Xn: Harmful; |
|---|---|
| Risk Phrases | R21/22 |
| Safety Phrases | S24/25 |
| WGK Germany | 1 |
| cerium oxalate decahydrate |
| Ceriumoxalatenonahydrate |
| decahydrate cerium oxalate |
| Cerium sesquioxalate hydrate |