(1R,2S)-1-Amino-2,3-dihydro-1H-inden-2-ol
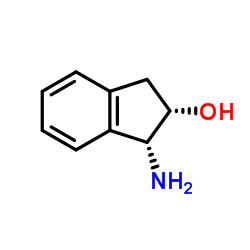
(1R,2S)-1-Amino-2,3-dihydro-1H-inden-2-ol structure
|
Common Name | (1R,2S)-1-Amino-2,3-dihydro-1H-inden-2-ol | ||
|---|---|---|---|---|
| CAS Number | 136030-00-7 | Molecular Weight | 149.19 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 290.0±40.0 °C at 760 mmHg | |
| Molecular Formula | C9H11NO | Melting Point | 117-121ºC | |
| MSDS | Chinese USA | Flash Point | 129.2±27.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of (1R,2S)-1-Amino-2,3-dihydro-1H-inden-2-ol(1R,2S)-1-Amino-2-indanol is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | (1R,2S)-(+)-1-Amino-2-Hydroxyindan |
|---|---|
| Synonym | More Synonyms |
| Description | (1R,2S)-1-Amino-2-indanol is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 290.0±40.0 °C at 760 mmHg |
| Melting Point | 117-121ºC |
| Molecular Formula | C9H11NO |
| Molecular Weight | 149.19 |
| Flash Point | 129.2±27.3 °C |
| Exact Mass | 149.084061 |
| PSA | 46.25000 |
| LogP | 0.43 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.626 |
| InChIKey | LOPKSXMQWBYUOI-DTWKUNHWSA-N |
| SMILES | NC1c2ccccc2CC1O |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2922199090 |
|
~33% 
(1R,2S)-1-Amino... CAS#:136030-00-7 |
| Literature: Sakurai, Rumiko; Sakai, Kenichi Tetrahedron Asymmetry, 2003 , vol. 14, # 4 p. 411 - 413 |
| Precursor 1 | |
|---|---|
| DownStream 2 | |
| HS Code | 2922199090 |
|---|---|
| Summary | 2922199090. other amino-alcohols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
A series of potent HIV-1 protease inhibitors containing a hydroxyethyl secondary amine transition state isostere: synthesis, enzyme inhibition, and antiviral activity.
J. Med. Chem. 35 , 2525, (1992) A series of HIV-1 protease inhibitors containing a novel hydroxyethyl secondary amine transition state isostere has been synthesized. The compounds exhibit a strong preference for the (R) stereochemis... |
|
|
Synthesis and antiviral activity of a series of HIV-1 protease inhibitors with functionality tethered to the P1 or P1' phenyl substituents: X-ray crystal structure assisted design.
J. Med. Chem. 35 , 1685, (1992) By tethering of a polar hydrophilic group to the P1 or P1' substituent of a Phe-based hydroxyethylene isostere, the antiviral potency of a series of HIV protease inhibitors was improved. The optimum e... |
|
|
HIV-1 protease inhibitors based on hydroxyethylene dipeptide isosteres: an investigation into the role of the P1' side chain on structure-activity.
J. Med. Chem. 35 , 1702, (1992) A systematic investigation was undertaken to determine the role of the P1' sidechain in a series of hydroxyethylene isostere based inhibitors of HIV-1 protease. Substitution and homologation of the be... |
| (1R,2S)-1-amino-2,3-dihydro-1H-inden-2-ol |
| (1R,2S)-1-Aminoindan-2-ol |
| MFCD00216656 |
| (1R,2S)-(+)-cis-1-Amino-2-hydroxyindane |
| 1H-Inden-2-ol, 1-amino-2,3-dihydro-, (1R,2S)- |
| (1R,2S)-(+)-1-Amino-2-indanol |
| (1R,2S)-(+)-1-Amino-2-hydroxyindan |
| (1R,2S)-(+)-cis-1-amino-2-indanol |
| (1R,2S)-1-Amino-2-indanol |
| Cis-(1R,2S)-1-amino-2-indanol |
![BIS((3AR,8AS)-8,8A-DIHYDRO-3AH-INDENO[1,2-D]OXAZOL-2-YL)METHANE structure](https://image.chemsrc.com/caspic/314/180186-94-1.png) CAS#:180186-94-1
CAS#:180186-94-1 CAS#:766556-66-5
CAS#:766556-66-5
