PRT-2607
Modify Date: 2025-09-09 13:04:27
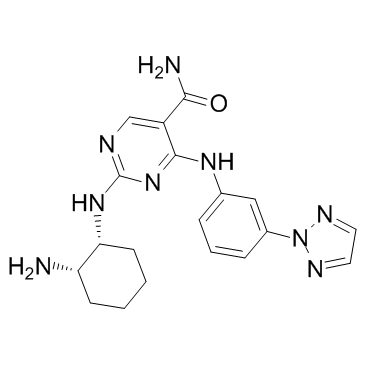
PRT-2607 structure
|
Common Name | PRT-2607 | ||
|---|---|---|---|---|
| CAS Number | 1370261-96-3 | Molecular Weight | 393.446 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 707.6±70.0 °C at 760 mmHg | |
| Molecular Formula | C19H23N9O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 381.7±35.7 °C | |
Use of PRT-2607PRT062607(P505-15; PRT-2607; BIIB-057) is a highly specific and potent inhibitor of Syk with IC50 of 1-2 nM; >80-fold selective for Syk than Fgr, Lyn, FAK, Pyk2 and Zap70.IC50 value: 1-2 nM [1]Target:Syk kinase inhibitorin vitro: In human whole blood, P505-15 potently inhibited B cell antigen receptor-mediated B cell signaling and activation (IC50 0.27 and 0.28 μM, respectively) and Fcε receptor 1-mediated basophil degranulation (IC50 0.15 μM) [1]. P505-15 successfully inhibited SYK-mediated B-cell receptor signaling and decreased cell viability in NHL and CLL [2]. PRT318 and P505-15 effectively antagonize CLL cell survival after BCR triggering and in nurse-like cell-co-cultures. Moreover, they inhibit BCR-dependent secretion of the chemokines CCL3 and CCL4 by CLL cells, and leukemia cell migration toward the tissue homing chemokines CXCL12, CXCL13, and beneath stromal cells. PRT318 and P505-15 furthermore inhibit Syk and extracellular signal-regulated kinase phosphorylation after BCR triggering [3].in vivo: Similar levels of ex vivo inhibition were measured after dosing in mice (Syk signaling IC50 0.32 μM). Oral administration of P505-15 produced dose-dependent anti-inflammatory activity in two rodent models of rheumatoid arthritis [1]. Oral dosing in mice prevented BCR-mediated splenomegaly and significantly inhibited NHL tumor growth in a xenograft model. In addition, combination treatment of primary CLL cells with P505-15 plus fludarabine produced synergistic enhancement of activity at nanomolar concentrations [2]. |
| Name | prt062607 |
|---|---|
| Synonym | More Synonyms |
| Description | PRT062607(P505-15; PRT-2607; BIIB-057) is a highly specific and potent inhibitor of Syk with IC50 of 1-2 nM; >80-fold selective for Syk than Fgr, Lyn, FAK, Pyk2 and Zap70.IC50 value: 1-2 nM [1]Target:Syk kinase inhibitorin vitro: In human whole blood, P505-15 potently inhibited B cell antigen receptor-mediated B cell signaling and activation (IC50 0.27 and 0.28 μM, respectively) and Fcε receptor 1-mediated basophil degranulation (IC50 0.15 μM) [1]. P505-15 successfully inhibited SYK-mediated B-cell receptor signaling and decreased cell viability in NHL and CLL [2]. PRT318 and P505-15 effectively antagonize CLL cell survival after BCR triggering and in nurse-like cell-co-cultures. Moreover, they inhibit BCR-dependent secretion of the chemokines CCL3 and CCL4 by CLL cells, and leukemia cell migration toward the tissue homing chemokines CXCL12, CXCL13, and beneath stromal cells. PRT318 and P505-15 furthermore inhibit Syk and extracellular signal-regulated kinase phosphorylation after BCR triggering [3].in vivo: Similar levels of ex vivo inhibition were measured after dosing in mice (Syk signaling IC50 0.32 μM). Oral administration of P505-15 produced dose-dependent anti-inflammatory activity in two rodent models of rheumatoid arthritis [1]. Oral dosing in mice prevented BCR-mediated splenomegaly and significantly inhibited NHL tumor growth in a xenograft model. In addition, combination treatment of primary CLL cells with P505-15 plus fludarabine produced synergistic enhancement of activity at nanomolar concentrations [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 707.6±70.0 °C at 760 mmHg |
| Molecular Formula | C19H23N9O |
| Molecular Weight | 393.446 |
| Flash Point | 381.7±35.7 °C |
| Exact Mass | 393.202545 |
| PSA | 149.66000 |
| LogP | 0.74 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.783 |
| InChIKey | TXGKRVFSSHPBAJ-JKSUJKDBSA-N |
| SMILES | NC(=O)c1cnc(NC2CCCCC2N)nc1Nc1cccc(-n2nccn2)c1 |
| Storage condition | 2-8℃ |
| PBN20121121 |
| 2-{[(1R,2S)-2-Aminocyclohexyl]amino}-4-{[3-(2H-1,2,3-triazol-2-yl)phenyl]amino}-5-pyrimidinecarboxamide |
| P505-15 HCl |
| 5-Pyrimidinecarboxamide, 2-[[(1R,2S)-2-aminocyclohexyl]amino]-4-[[3-(2H-1,2,3-triazol-2-yl)phenyl]amino]- |
| p50515/p505-15 |