Ribociclib succinate hydrate
Modify Date: 2025-08-25 07:57:22
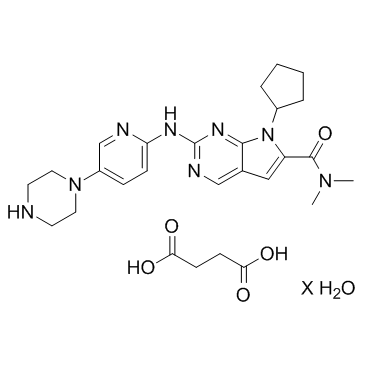
Ribociclib succinate hydrate structure
|
Common Name | Ribociclib succinate hydrate | ||
|---|---|---|---|---|
| CAS Number | 1374639-79-8 | Molecular Weight | 570.641 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C27H38N8O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Ribociclib succinate hydrateRibociclib succinate hydrate (LEE011 succinate hydrate) is a highly specific CDK4/6 inhibitor with IC50 values of 10 nM and 39 nM, respectively, and is over 1,000-fold less potent against the cyclin B/CDK1 complex. |
| Name | LEE011 succinate hydrate |
|---|---|
| Synonym | More Synonyms |
| Description | Ribociclib succinate hydrate (LEE011 succinate hydrate) is a highly specific CDK4/6 inhibitor with IC50 values of 10 nM and 39 nM, respectively, and is over 1,000-fold less potent against the cyclin B/CDK1 complex. |
|---|---|
| Related Catalog | |
| Target |
CDK4:10 nM (IC50) CDK6:39 nM (IC50) |
| In Vitro | Treating a panel of 17 neuroblastoma cell lines with Ribociclib (LEE011) across a four-log dose range (10 to 10,000 nM). Treatment with Ribociclib significantly inhibits substrate adherent growth relative to the control in 12 of the 17 neuroblastoma cell lines examined (mean IC50=306±68 nM, considering sensitive lines only, where sensitivity is defined as an IC50 of less than 1 μM. Ribociclib treatment of two neuroblastoma cell lines (BE2C and IMR5) with demonstrated sensitivity to CDK4/6 inhibition results in a dose-dependent accumulation of cells in the G0/G1 phase of the cell cycle. This G0/G1 arrest becomes significant at Ribociclib concentrations of 100 nM (p=0.007) and 250 nM (p=0.01), respectively[2]. |
| In Vivo | CB17 immunodeficient mice bearing BE2C, NB-1643 (MYCN amplified, sensitive in vitro), or EBC1 (non-amplified, resistant in vitro) xenografts are treated once daily for 21 days with Ribociclib (LEE011; 200 mg/kg) or with a vehicle control. This dosing strategy is well tolerated, as no weight loss or other signs of toxicity are observed in any of the xenograft models. Tumor growth is significantly delayed throughout the 21 days of treatment in mice harboring the BE2C or 1643 xenografts (both, p<0.0001), although growth resumed post-treatment[2]. |
| Cell Assay | Cells are grown for 24 hours in 35 mm plates, treated with 500 nM Ribociclib for 6 days, and then fixed and stained overnight. Cells are then imaged for SA-β-gal using an Axio Observer D.1 phase contrast microscope. The percentage of SA-β-gal positive cells is determined by counting the number of positive cells present in three separate microscope frames, and then normalizing to the control. To assess apoptotic activity, cells are plated in triplicate in 96 well plates, treated with Ribociclib, and assayed for caspase 3/7 activation 16 hours after treatment with Caspase-Glo 3/7. Cells treated with SN-38 are used as a positive control[2]. |
| Animal Admin | Mice[2] The BE2C, NB-1643, or EBC1 cell line-derived xenografts are implanted subcutaneously into the right flank of CB17 SCID-/- mice. Animals bearing engrafted tumors of 200-600 mm3 are then randomized to oral treatment with 200 mg/kg Ribociclib in 0.5 % methylcellulose (n=10) or vehicle (n=10) daily for a total of 21 days. Tumor burden is determined periodically throughout treatment according to the formula (π/6)×d2, where d represents the mean tumor diameter obtained by caliper measurement. |
| References |
| Molecular Formula | C27H38N8O6 |
|---|---|
| Molecular Weight | 570.641 |
| Exact Mass | 570.291443 |
| 7H-Pyrrolo[2,3-d]pyrimidine-6-carboxamide, 7-cyclopentyl-N,N-dimethyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]-, butanedioate, hydrate (1:1:1) |
| Succinic acid - 7-cyclopentyl-N,N-dimethyl-2-{[5-(1-piperazinyl)-2-pyridinyl]amino}-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide hydrate (1:1:1) |
| LEE011 (succinate hydrate) |