potassium ethylxanthate
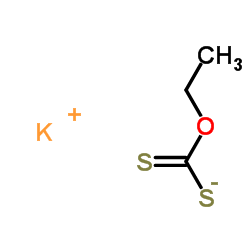
potassium ethylxanthate structure
|
Common Name | potassium ethylxanthate | ||
|---|---|---|---|---|
| CAS Number | 140-89-6 | Molecular Weight | 160.299 | |
| Density | 1,558 g/cm3 | Boiling Point | 120.5ºC at 760 mmHg | |
| Molecular Formula | C3H5KOS2 | Melting Point | 209-214 °C | |
| MSDS | Chinese USA | Flash Point | 96°C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
| Name | potassium ethylxanthate |
|---|---|
| Synonym | More Synonyms |
| Density | 1,558 g/cm3 |
|---|---|
| Boiling Point | 120.5ºC at 760 mmHg |
| Melting Point | 209-214 °C |
| Molecular Formula | C3H5KOS2 |
| Molecular Weight | 160.299 |
| Flash Point | 96°C |
| Exact Mass | 159.941895 |
| PSA | 66.62000 |
| LogP | 1.50530 |
| Vapour Pressure | 18.2mmHg at 25°C |
| Stability | Stable. Incompatible with strong acids, strong bases, strong oxidizing agents. |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H228-H302 + H332-H315-H319-H335 |
| Supplemental HS | In use may form flammable/explosive vapour-air mixture. |
| Precautionary Statements | P210-P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S37/39-S26-S37 |
| RIDADR | 3342 |
| RTECS | FG1575000 |
| Packaging Group | II |
| Hazard Class | 4.2 |
| HS Code | 2930909090 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2905199090 |
|---|---|
| Summary | 2905199090. saturated monohydric alcohols. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Time, illumination and solvent dependent stability of cadmium sulfide nanoparticle suspensions.
J. Colloid. Interface Sci. 430 , 283-92, (2014) The optical properties of cadmium sulfide (CdS) nanoparticles in suspension are affected by morphology and suspending solvent. Time dependent stability of these properties is solvent dependent and is ... |
|
|
A novel method of extracting plasmid DNA from Helicobacter species.
Helicobacter 3(4) , 269-77, (1998) Plasmids are extra-chromosomal DNA that may encode products that aid in virulence, pathogenesis, and the spread of antibiotic resistance among a wide spectrum of bacteria. Plasmids have been detected ... |
|
|
Effects of lipophilic complex formation on the disposition of nickel in experimental animals.
Sci. Total Environ. 148(2-3) , 217-42, (1994) Dithiocarbamates, thiuram sulphides, xanthates, pyridinethiones and halogenated 8-hydroxyquinolines are groups of compounds which can form lipophilic complexes with Ni2+. These compounds are widely us... |
| Carbonic acid, dithio-, O-ethyl ester, potassium salt |
| potassium,ethoxymethanedithioate |
| EINECS 205-439-3 |
| Potassium Xanthogenate |
| DITHIOCARBONIC ACID O-ETHYL ESTER,POTASSIUM SALT DIMER |
| Potassium ethyl xanthogenate |
| Ethyl potassium xanthate |
| Potassium O-ethyl carbonodithioate |
| Potassium O-ethyl dithiocarbonate |
| potassium O-ethyl xanthate |
| Potassium ethyl xanthate |
| Ethylxanthic acid potassium salt |
| Carbonodithioic acid O-ethyl ester potassium salt |
| POTASSIUM ETHYLXANTHATE |
| O-ethyl potassium carbonodithioate |
| xanthic acid, ethyl-, potassium salt |
| MFCD00004931 |
| Carbonodithioic acid, O-ethyl ester, potassium salt (1:1) |
| Carbonodithioic acid, O-ethyl ester, potassium salt |
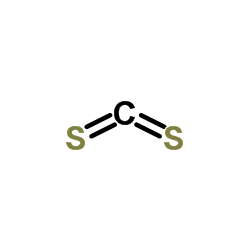 CAS#:75-15-0
CAS#:75-15-0 CAS#:64-17-5
CAS#:64-17-5 CAS#:917-58-8
CAS#:917-58-8 CAS#:2314-48-9
CAS#:2314-48-9 CAS#:2905-52-4
CAS#:2905-52-4 CAS#:60-29-7
CAS#:60-29-7 CAS#:502-55-6
CAS#:502-55-6 CAS#:465546-82-1
CAS#:465546-82-1 CAS#:583-39-1
CAS#:583-39-1![5-METHOXYBENZO[D]THIAZOLE-2-THIOL structure](https://image.chemsrc.com/caspic/420/55690-60-3.png) CAS#:55690-60-3
CAS#:55690-60-3![5-bromobenzo[d]oxazole-2-thiol structure](https://image.chemsrc.com/caspic/045/439607-87-1.png) CAS#:439607-87-1
CAS#:439607-87-1 CAS#:37052-78-1
CAS#:37052-78-1 CAS#:58089-25-1
CAS#:58089-25-1 CAS#:34126-43-7
CAS#:34126-43-7 CAS#:332-51-4
CAS#:332-51-4 CAS#:49559-83-3
CAS#:49559-83-3![5-FLUORO-1H-BENZO[D]IMIDAZOLE-2(3H)-THIONE structure](https://image.chemsrc.com/caspic/400/583-42-6.png) CAS#:583-42-6
CAS#:583-42-6
