Tin-protoporphyrin IX
Modify Date: 2025-08-25 10:23:42
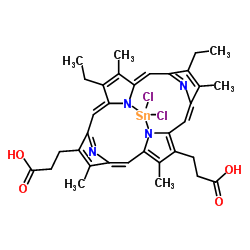
Tin-protoporphyrin IX structure
|
Common Name | Tin-protoporphyrin IX | ||
|---|---|---|---|---|
| CAS Number | 14325-05-4 | Molecular Weight | 754.290 | |
| Density | N/A | Boiling Point | 849.3±75.0 °C at 760 mmHg | |
| Molecular Formula | C34H32Cl2N4O4Sn | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 467.5±37.1 °C | |
Use of Tin-protoporphyrin IXTin-protoporphyrin IX (SnPPIX) is a potent Heme oxygenase-1 (HO-1) inhibitor. Tin-protoporphyrin IX (SnPPIX) sensitizes pancreatic ductal adenocarcinoma (PDAC) tumors to chemotherapy in mice model[1]. |
| Name | tin protoporphyrin IX dichloride |
|---|---|
| Synonym | More Synonyms |
| Description | Tin-protoporphyrin IX (SnPPIX) is a potent Heme oxygenase-1 (HO-1) inhibitor. Tin-protoporphyrin IX (SnPPIX) sensitizes pancreatic ductal adenocarcinoma (PDAC) tumors to chemotherapy in mice model[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: Heme oxygenase-1 (HO-1)[1] |
| In Vitro | Tin-protoporphyrin IX (SnPPIX)(20 μM, 50 μM; 24 hours) significantly suppressed the proliferation of Capan-1 and CD18/HPAF cells. In contrast, SnPPIX has no significant effect on PDAC cells proliferation at all exposures except at 50 μM[1]. Cell Viability Assay[1] Cell Line: Capan-1, CD18/HPAF, PDAC cells Concentration: 20 μM, 50 μM Incubation Time: 24 or 72 hours Result: Inhibited Capan-1 and CD18/HPAF cells proliferation and inhibits PDAC cells growth at 50μM. |
| In Vivo | Tin-protoporphyrin IX (SnPPIX)(intraperitoneal injection; 5 mg/kg; at 0, 7, 15, and 20 days) alone or combines with Gemcitabine significantly reduced the weight of pancreatic tumors (P < 0.05), decreases metastasis and improved the efficacy of Gemcitabine treatment[1]. Animal Model: Male and female athymic nude mice with PDAC cell-derived xenograft tumors[1] Dosage: 5 mg/kg Administration: Intraperitoneal injection; at days 1, 4, 6, 8, 11, 13, 15, 18, and 20 Result: Inhibited tumor growth and sensitized tumors to chemotherapy (Gemcitabine). |
| References |
| Boiling Point | 849.3±75.0 °C at 760 mmHg |
|---|---|
| Molecular Formula | C34H32Cl2N4O4Sn |
| Molecular Weight | 754.290 |
| Flash Point | 467.5±37.1 °C |
| Exact Mass | 754.113586 |
| PSA | 109.18000 |
| LogP | 4.75380 |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| 3,3'-[(1Z,6Z,12Z,17Z)-22,22-Dichloro-15,20-diethyl-5,9,14,19-tetramethyl-21,23,24,25-tetraaza-22-stannahexacyclo[9.9.3.1.1.0.0]pentacosa-1,3(25),4,6,8,10,12,14,16(24),17,19-un 
decaene-4,10-diyl]dipropanoic acid |
| tin protophorphyrin |
 CAS#:106344-20-1
CAS#:106344-20-1