UPF-648 sodium salt
Modify Date: 2025-09-17 20:39:14
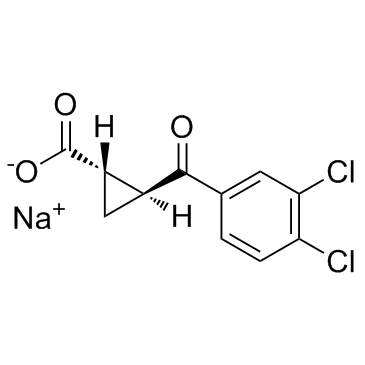
UPF-648 sodium salt structure
|
Common Name | UPF-648 sodium salt | ||
|---|---|---|---|---|
| CAS Number | 1465017-87-1 | Molecular Weight | 281.067 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C11H7Cl2NaO3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of UPF-648 sodium saltUPF-648 sodium salt is a potent kynurenine 3-monooxygenase (KMO) inhibitor; exhibits highly active at 1 uM (81 ± 10% KMO inhibition); ineffective at blocking KAT activity.IC50 value: 1 uM(81 ± 10 % inhibition) [1]Target: KMO inhibitorin vitro: BFF 122 inhibited KAT activity almost completely at both 1 and 0.1 mM. The effect was still remarkable at 0.01 mM (70 ± 1 % inhibition). At the same three concentrations, BFF 122 did not affect KMO activity significantly. In contrast, UPF 648 totally blocked KMO at 0.1 and 0.01 mM and was still highly active at 0.001 mM (81 ± 10 % inhibition), but the compound was essentially ineffective at blocking KAT activity [1]. UPF 648 binds close to the FAD cofactor and perturbs the local active-site structure, preventing productive binding of the substrate l-kynurenine. Functional assays and targeted mutagenesis reveal that the active-site architecture and UPF 648 binding are essentially identical in human KMO, validating the yeast KMO-UPF 648 structure as a template for structure-based drug design [3].in vivo: Applying an identical experimental design, separate rats were used to study the effect of KMO inhibition on the de novo synthesis of KP metabolites in the lesioned striatum. These animals were bilaterally injected with 0.1 mM UPF 648 and 3H-kynurenine in PBS. 0.1 mM UPF 648 significantly reduced the neosynthesis of 3-HK and QUIN in the lesioned striatum (by 77 % and 66%, respectively) and moderately (27%) but significantly increased the de novo formation of KYNA [1]. Administered to pregnant rats or mice on the last day of gestation, UPF 648 (50 mg/kg, i.p.) produced qualitatively similar changes (i.e., large increases in kynurenine and KYNA and reductions in 3-HK and QUIN) in the brain and liver of the offspring. Rat pups delivered by UPF 648-treated mothers and immediately exposed to neonatal asphyxia showed further enhanced brain KYNA levels [2]. UPF 648, has an IC50 of 20 nM and provides protection against intrastriatal QUIN injections in kynurenine aminotransferase (KAT II) deficient mice. UPF 648 treatment also shifts KP metabolism towards enhanced neuroprotective KYNA formation [3]. |
| Name | UPF-648 sodium salt |
|---|---|
| Synonym | More Synonyms |
| Description | UPF-648 sodium salt is a potent kynurenine 3-monooxygenase (KMO) inhibitor; exhibits highly active at 1 uM (81 ± 10% KMO inhibition); ineffective at blocking KAT activity.IC50 value: 1 uM(81 ± 10 % inhibition) [1]Target: KMO inhibitorin vitro: BFF 122 inhibited KAT activity almost completely at both 1 and 0.1 mM. The effect was still remarkable at 0.01 mM (70 ± 1 % inhibition). At the same three concentrations, BFF 122 did not affect KMO activity significantly. In contrast, UPF 648 totally blocked KMO at 0.1 and 0.01 mM and was still highly active at 0.001 mM (81 ± 10 % inhibition), but the compound was essentially ineffective at blocking KAT activity [1]. UPF 648 binds close to the FAD cofactor and perturbs the local active-site structure, preventing productive binding of the substrate l-kynurenine. Functional assays and targeted mutagenesis reveal that the active-site architecture and UPF 648 binding are essentially identical in human KMO, validating the yeast KMO-UPF 648 structure as a template for structure-based drug design [3].in vivo: Applying an identical experimental design, separate rats were used to study the effect of KMO inhibition on the de novo synthesis of KP metabolites in the lesioned striatum. These animals were bilaterally injected with 0.1 mM UPF 648 and 3H-kynurenine in PBS. 0.1 mM UPF 648 significantly reduced the neosynthesis of 3-HK and QUIN in the lesioned striatum (by 77 % and 66%, respectively) and moderately (27%) but significantly increased the de novo formation of KYNA [1]. Administered to pregnant rats or mice on the last day of gestation, UPF 648 (50 mg/kg, i.p.) produced qualitatively similar changes (i.e., large increases in kynurenine and KYNA and reductions in 3-HK and QUIN) in the brain and liver of the offspring. Rat pups delivered by UPF 648-treated mothers and immediately exposed to neonatal asphyxia showed further enhanced brain KYNA levels [2]. UPF 648, has an IC50 of 20 nM and provides protection against intrastriatal QUIN injections in kynurenine aminotransferase (KAT II) deficient mice. UPF 648 treatment also shifts KP metabolism towards enhanced neuroprotective KYNA formation [3]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C11H7Cl2NaO3 |
|---|---|
| Molecular Weight | 281.067 |
| Exact Mass | 279.966980 |
| InChIKey | DBXRQPXNFMACQA-LEUCUCNGSA-M |
| SMILES | O=C([O-])C1CC1C(=O)c1ccc(Cl)c(Cl)c1.[Na+] |
| Storage condition | 2-8℃ |
| Cyclopropanecarboxylic acid, 2-(3,4-dichlorobenzoyl)-, sodium salt, (1S,2S)- (1:1) |
| Sodium (1S,2S)-2-(3,4-dichlorobenzoyl)cyclopropanecarboxylate |
| UPF-648 (sodium salt) |