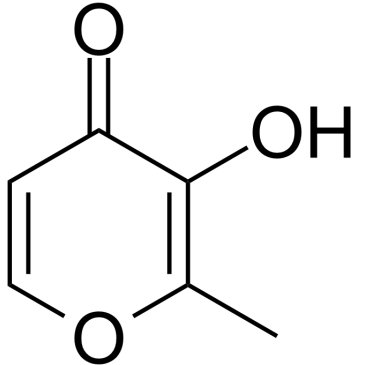
Aspalatone
Modify Date: 2025-08-20 17:56:35
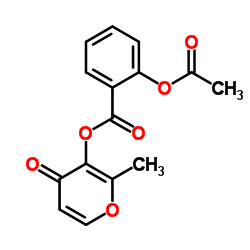
Aspalatone structure
|
Common Name | Aspalatone | ||
|---|---|---|---|---|
| CAS Number | 147249-33-0 | Molecular Weight | 288.252 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 466.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C15H12O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 209.0±28.8 °C | |
Use of AspalatoneAspalatone is an anti-platelet aggregator (antithrombotic) that has been shown to prolong bleeding time in the mouse model. Aspalatone generates antioxidant and neuroprotective effects against kainic acid-induced epilepsy. |
| Name | (2-methyl-4-oxopyran-3-yl) 2-acetyloxybenzoate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 466.1±45.0 °C at 760 mmHg |
| Molecular Formula | C15H12O6 |
| Molecular Weight | 288.252 |
| Flash Point | 209.0±28.8 °C |
| Exact Mass | 288.063385 |
| PSA | 82.81000 |
| LogP | 1.16 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.579 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
Aspalatone CAS#:147249-33-0 |
| Literature: Han; Suh; Yang; Park; Kang; Kim Arzneimittel-Forschung/Drug Research, 1994 , vol. 44, # 10 p. 1122 - 1126 |
|
~% 
Aspalatone CAS#:147249-33-0 |
| Literature: Han; Suh; Yang; Park; Kang; Kim Arzneimittel-Forschung/Drug Research, 1994 , vol. 44, # 10 p. 1122 - 1126 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| Aspalatone |
| 2-Methyl-4-oxo-4H-pyran-3-yl 2-acetoxybenzoate |
| BK-111 |
| Benzoic acid, 2-(acetyloxy)-, 2-methyl-4-oxo-4H-pyran-3-yl ester |
| acetyl salicylic acid maltol ester |