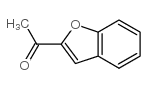
Benzarone
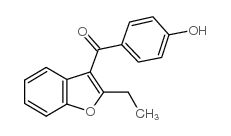
Benzarone structure
|
Common Name | Benzarone | ||
|---|---|---|---|---|
| CAS Number | 1477-19-6 | Molecular Weight | 266.29100 | |
| Density | 1.234g/cm3 | Boiling Point | 473.6ºC at 760mmHg | |
| Molecular Formula | C17H14O3 | Melting Point | 124.3° | |
| MSDS | N/A | Flash Point | 240.2ºC | |
Use of BenzaroneBenzarone (Fragivix) is a potent human uric acid transporter 1 (hURAT1) inhibitor, with an IC50 of 2.8 μM in oocyte. Benzarone could lower uric acid serum levels[1]. |
| Name | (2-ethyl-1-benzofuran-3-yl)-(4-hydroxyphenyl)methanone |
|---|---|
| Synonym | More Synonyms |
| Description | Benzarone (Fragivix) is a potent human uric acid transporter 1 (hURAT1) inhibitor, with an IC50 of 2.8 μM in oocyte. Benzarone could lower uric acid serum levels[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 2.8 μM (hURAT1 in oocyte)[1] |
| References |
| Density | 1.234g/cm3 |
|---|---|
| Boiling Point | 473.6ºC at 760mmHg |
| Melting Point | 124.3° |
| Molecular Formula | C17H14O3 |
| Molecular Weight | 266.29100 |
| Flash Point | 240.2ºC |
| Exact Mass | 266.09400 |
| PSA | 50.44000 |
| LogP | 3.93180 |
| Vapour Pressure | 1.36E-09mmHg at 25°C |
| Index of Refraction | 1.638 |
| InChIKey | RFRXIWQYSOIBDI-UHFFFAOYSA-N |
| SMILES | CCc1oc2ccccc2c1C(=O)c1ccc(O)cc1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2932999099 |
|---|
|
~90% 
Benzarone CAS#:1477-19-6 |
| Literature: J-PHARMA CO., LTD.; WEMPE, Michael, F.; ENDOU, Hitoshi Patent: WO2012/48058 A2, 2012 ; Location in patent: Page/Page column 14-15 ; WO 2012/048058 A2 |
|
~% 
Benzarone CAS#:1477-19-6 |
| Literature: Bulletin de la Societe Chimique de France, , p. 1682,1685 |
|
~% 
Benzarone CAS#:1477-19-6 |
| Literature: Journal of the Chemical Society, , p. 625,627 |
|
~% 
Benzarone CAS#:1477-19-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 54, # 8 p. 2701 - 2713 |
|
~% 
Benzarone CAS#:1477-19-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 54, # 8 p. 2701 - 2713 |
|
~% 
Benzarone CAS#:1477-19-6 |
| Literature: WO2012/48058 A2, ; WO 2012/048058 A2 |
|
~% 
Benzarone CAS#:1477-19-6 |
| Literature: Bulletin de la Societe Chimique de France, , p. 1682,1685 |
|
~% 
Benzarone CAS#:1477-19-6 |
| Literature: Bulletin de la Societe Chimique de France, , p. 1682,1685 |
|
~% 
Benzarone CAS#:1477-19-6 |
| Literature: Bulletin de la Societe Chimique de France, , p. 1682,1685 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Interactions of urate transporter URAT1 in human kidney with uricosuric drugs.
Nephrology (Carlton.) 16(2) , 156-62, (2011) Hyperuricaemia is a significant factor in a variety of diseases, including gout and cardiovascular diseases. The kidney plays a dominant role in maintaining plasma urate levels through the excretion p... |
|
|
The EYA tyrosine phosphatase activity is pro-angiogenic and is inhibited by benzbromarone.
PLoS ONE 7(4) , e34806, (2012) Eyes Absents (EYA) are multifunctional proteins best known for their role in organogenesis. There is accumulating evidence that overexpression of EYAs in breast and ovarian cancers, and in malignant p... |
|
|
[Effect of benzarone on the oxygen consumption and the mechanical activity of vascular smooth muscle].
Arzneimittelforschung 33(2) , 211-4, (1983) The influence of 2-ethyl-3-(4-hydroxy-benzoyl)-benzofurane (benzarone, Fragivic) on the energy metabolism of the wall of the rabbit common carotid artery and the rat portal vein was investigated by me... |
| (2-Ethylbenzofuran-3-yl)(4-hydroxyphenyl)methanone |
| Fragivix |
| Benzarone |
| (2-ETHYL-1-BENZOFURAN-3-YL)(4-HYDROXYPHENYL)METHANONE |
| Fagivil |
| Vasoc |
| Fragivil |
| Benzaron |
| Venagil |

 structure](https://image.chemsrc.com/caspic/225/90908-77-3.png)