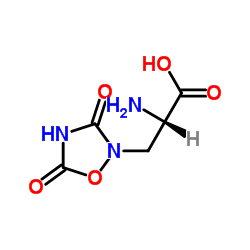
L-Albizziin

L-Albizziin structure
|
Common Name | L-Albizziin | ||
|---|---|---|---|---|
| CAS Number | 1483-07-4 | Molecular Weight | 147.13300 | |
| Density | 1.43 g/cm3 | Boiling Point | 359.1ºC at 760 mmHg | |
| Molecular Formula | C4H9N3O3 | Melting Point | 217°C (dec.) | |
| MSDS | Chinese USA | Flash Point | 171ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of L-AlbizziinL-albizziin, as a sulfhydryl group reagent, is a glutamase inhibitor. L-albizziin can be used for the research of cancer[1]. |
| Name | Albizziin |
|---|---|
| Synonym | More Synonyms |
| Description | L-albizziin, as a sulfhydryl group reagent, is a glutamase inhibitor. L-albizziin can be used for the research of cancer[1]. |
|---|---|
| Related Catalog | |
| Target |
Glutamase[1] |
| In Vitro | L-albizziin, as a sulfhydryl group reagent, is a glutamase inhibitor[1]. |
| References |
| Density | 1.43 g/cm3 |
|---|---|
| Boiling Point | 359.1ºC at 760 mmHg |
| Melting Point | 217°C (dec.) |
| Molecular Formula | C4H9N3O3 |
| Molecular Weight | 147.13300 |
| Flash Point | 171ºC |
| Exact Mass | 147.06400 |
| PSA | 118.44000 |
| Vapour Pressure | 3.95E-06mmHg at 25°C |
| Index of Refraction | 1.55 |
| InChIKey | GZYFIMLSHBLMKF-REOHCLBHSA-N |
| SMILES | NC(=O)NCC(N)C(=O)O |
| Storage condition | -20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2924199090 |
|
~68% 
L-Albizziin CAS#:1483-07-4 |
| Literature: Piper, J. R.; McCaleb, G. S.; Montgomery, J. A.; Schmid, F. A.; Sirotnak, F. M. Journal of Medicinal Chemistry, 1985 , vol. 28, # 8 p. 1016 - 1025 |
|
~% 
L-Albizziin CAS#:1483-07-4 |
| Literature: Journal of the Chemical Society, Chemical Communications, , # 5 p. 256 - 257 |
|
~% 
L-Albizziin CAS#:1483-07-4 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 23, # 11 p. 2669 - 2677 |
|
~% 
L-Albizziin CAS#:1483-07-4 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 23, # 11 p. 2669 - 2677 |
|
~% 
L-Albizziin CAS#:1483-07-4 |
| Literature: Acta Chemica Scandinavica (1947-1973), , vol. 13, p. 1565,1572 |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Molecular and genetic characterization of human cell lines resistant to L-asparaginase and albizziin.
Somat. Cell Mol. Genet. 16(1) , 59-65, (1990) Human cell lines resistant to L-asparaginase or albizziin were isolated by multistep selection of HT1080 fibrosarcoma and MIA PaCa-2 pancreatic carcinoma cells. Mutants were cross-resistant to both dr... |
|
|
Chromosomal alterations associated with overproduction of asparagine synthetase in albizziin-resistant Chinese hamster ovary cells.
Mol. Cell. Biol. 3(3) , 391-8, (1983) The amino acid analog albizziin was used to isolate Chinese hamster ovary cell lines which overproduce asparagine synthetase. Mutants selected in a single step after ethyl methane sulfonate mutagenesi... |
|
|
Use of the Escherichia coli gene for asparagine synthetase as a selective marker in a shuttle vector capable of dominant transfection and amplification in animal cells.
Mol. Cell. Biol. 7(5) , 1623-8, (1987) A new dominant amplifiable selective system for use in bacterium-animal cell shuttle vectors was developed by the insertion of a 2-kilobase genomic fragment containing the cloned Escherichia coli gene... |
| MFCD00007952 |
| L-b-Ureidoalanine |
| L-albizziine |
| L-ALBIZZIIN |
| EINECS 216-046-1 |