Pregabalin

Pregabalin structure
|
Common Name | Pregabalin | ||
|---|---|---|---|---|
| CAS Number | 148553-50-8 | Molecular Weight | 159.226 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 274.0±23.0 °C at 760 mmHg | |
| Molecular Formula | C8H17NO2 | Melting Point | 194-196ºC | |
| MSDS | Chinese USA | Flash Point | 119.5±22.6 °C | |
| Symbol |


GHS05, GHS08 |
Signal Word | Danger | |
| Name | Pregabalin |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 274.0±23.0 °C at 760 mmHg |
| Melting Point | 194-196ºC |
| Molecular Formula | C8H17NO2 |
| Molecular Weight | 159.226 |
| Flash Point | 119.5±22.6 °C |
| Exact Mass | 159.125931 |
| PSA | 63.32000 |
| LogP | 1.12 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.465 |
| InChIKey | AYXYPKUFHZROOJ-ZETCQYMHSA-N |
| SMILES | CC(C)CC(CN)CC(=O)O |
| Storage condition | Store at RT |
| Symbol |


GHS05, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H318-H361 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Hazard Codes | Xn,T,F |
| Risk Phrases | 63-48/22-39/23/24/25-23/24/25-11 |
| Safety Phrases | 22-36/37-45-16-7 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2915900090 |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |
|
Development of a SPE-HPLC-MS/MS method for the determination of most prescribed pharmaceuticals and related metabolites in urban sewage samples.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 990 , 23-30, (2015) Based on regional prescription data several pharmaceuticals with variable amounts of prescription and corresponding metabolites were selected and analyzed in influent and effluent samples of the sewag... |
|
|
Effect of the gastrointestinal prokinetic agent erythromycin on the pharmacokinetics of pregabalin controlled-release in healthy individuals: a phase I, randomized crossover trial.
Clin. Drug Investig. 35(5) , 299-305, (2015) The controlled-release (CR) formulation of pregabalin is designed to remain in the stomach for a prolonged period while slowly releasing pregabalin for absorption in the small intestine. This study ev... |
|
|
Evaluation of the matrix effect of different sample matrices for 33 pharmaceuticals by post-column infusion.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 1000 , 84-94, (2015) Matrix effects that occur during quantitative measurement by liquid chromatography mass spectrometry specifically when using electrospray ionization are a widely recognized phenomenon. Sample matrix c... |
| (S)-3-Aminomethyl-5-methyl-hexanoic acid |
| EINECS 200-659-6 |
| (S)-3-(Aminomethyl)-5-methylhexanoic acid CI-1008 PD-144723 |
| PREDNISOLONESODIUMPHOSPHATE |
| Hexanoic acid, 3-(aminomethyl)-5-methyl-, (3S)- |
| Pregablin |
| (S)-3-(Aminomethyl)-5-methylhexanoic acid |
| Pregabalin |
| Lyrica |
| prégabaline |
| (S)-3-Isobutyl GABA |
| MFCD00917044 |
| (S)-(+)-3-aminomethyl-5-methylhexanoic acid |
| (3S)-3-(Aminomethyl)-5-methylhexanoic acid |
 CAS#:181289-39-4
CAS#:181289-39-4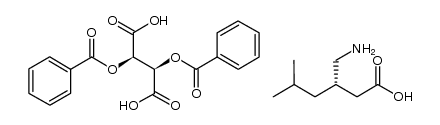 CAS#:1078737-39-9
CAS#:1078737-39-9 CAS#:1310495-04-5
CAS#:1310495-04-5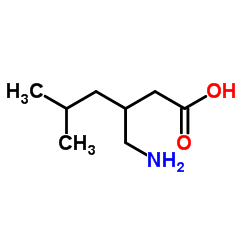 CAS#:128013-69-4
CAS#:128013-69-4 CAS#:181289-33-8
CAS#:181289-33-8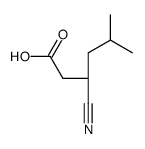 CAS#:181289-37-2
CAS#:181289-37-2
