Leachianone G
Modify Date: 2025-08-25 15:22:41
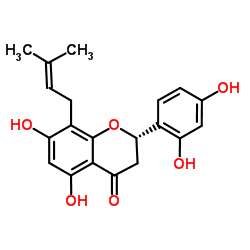
Leachianone G structure
|
Common Name | Leachianone G | ||
|---|---|---|---|---|
| CAS Number | 152464-78-3 | Molecular Weight | 356.369 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 639.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C20H20O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 231.4±25.0 °C | |
Use of Leachianone GLeachianone G is an antiviral flavonoid from the root bark of Morus alba L. Leachianone G shows potent antiviral activity against herpes simplex type 1 virus (HSV-1) with an IC50 value of 1.6 μg/mL[1]. |
| Name | Leachianone G |
|---|---|
| Synonym | More Synonyms |
| Description | Leachianone G is an antiviral flavonoid from the root bark of Morus alba L. Leachianone G shows potent antiviral activity against herpes simplex type 1 virus (HSV-1) with an IC50 value of 1.6 μg/mL[1]. |
|---|---|
| Related Catalog | |
| Target |
HSV-1:1.6 μg/mL (IC50) |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 639.6±55.0 °C at 760 mmHg |
| Molecular Formula | C20H20O6 |
| Molecular Weight | 356.369 |
| Flash Point | 231.4±25.0 °C |
| Exact Mass | 356.125977 |
| PSA | 107.22000 |
| LogP | 4.56 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.664 |
| Hazard Codes | Xi |
|---|
| 4H-1-Benzopyran-4-one, 2-(2,4-dihydroxyphenyl)-2,3-dihydro-5,7-dihydroxy-8-(3-methyl-2-butenyl)-, (2S)- |
| (2S)-2-(2,4-Dihydroxyphenyl)-5,7-dihydroxy-8-(3-methyl-2-buten-1-yl)-2,3-dihydro-4H-chromen-4-one |
| 4H-1-Benzopyran-4-one, 2-(2,4-dihydroxyphenyl)-2,3-dihydro-5,7-dihydroxy-8-(3-methyl-2-buten-1-yl)-, (2S)- |