Ethyl 5-methyl-1H-indole-2-carboxylate
Modify Date: 2025-08-27 06:27:32
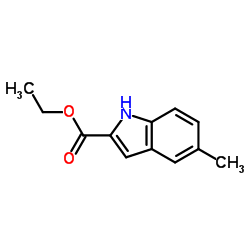
Ethyl 5-methyl-1H-indole-2-carboxylate structure
|
Common Name | Ethyl 5-methyl-1H-indole-2-carboxylate | ||
|---|---|---|---|---|
| CAS Number | 16382-15-3 | Molecular Weight | 203.237 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 356.5±22.0 °C at 760 mmHg | |
| Molecular Formula | C12H13NO2 | Melting Point | 162-164°C | |
| MSDS | Chinese USA | Flash Point | 169.4±22.3 °C | |
Use of Ethyl 5-methyl-1H-indole-2-carboxylateIt is an indole derivative. Indole ring system is an important building block or intermediate in the synthesis of many pharmaceutical agents. It is formed during the Fischer indolization of ethyl pyruvate 2-[2-(methanesulfonyloxy)-4-methyl]phenylhydrazine. |
| Name | Ethyl 5-methylindole-2-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 356.5±22.0 °C at 760 mmHg |
| Melting Point | 162-164°C |
| Molecular Formula | C12H13NO2 |
| Molecular Weight | 203.237 |
| Flash Point | 169.4±22.3 °C |
| Exact Mass | 203.094635 |
| PSA | 42.09000 |
| LogP | 3.56 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.609 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Substituent Effects in the Fischer Indolization of (2-Sulfonyloxyphenyl) hydrazones (Fischer Indolization and Related Compounds. XXX). Murakami Y, et al.
Chem. Pharm. Bull. 47 , 791-97, (1999)
|
| Ethyl 5-Methylindole-2-Carboxylate |
| Ethyl 5-methyl-1H-indole-2-carboxylate |
| MFCD00022703 |
| 1H-Indole-2-carboxylic acid, 5-methyl-, ethyl ester |
| 5-Methylindole-2-carboxylic acid ethyl ester |
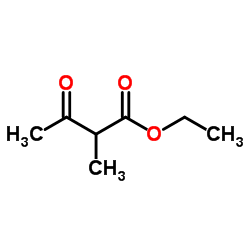 CAS#:609-14-3
CAS#:609-14-3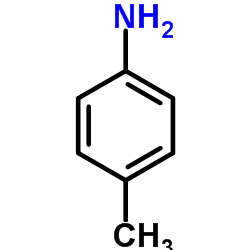 CAS#:106-49-0
CAS#:106-49-0![Propanoic acid, 2-[(4-methylphenyl)hydrazono]-, ethyl ester, (E) Structure](https://image.chemsrc.com/caspic/318/91462-34-9.png) CAS#:91462-34-9
CAS#:91462-34-9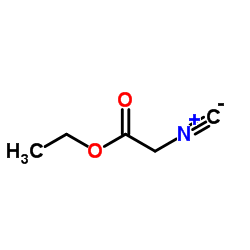 CAS#:2999-46-4
CAS#:2999-46-4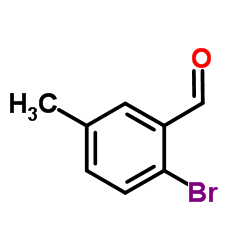 CAS#:90221-55-9
CAS#:90221-55-9 CAS#:64-17-5
CAS#:64-17-5![2-[(4-methylphenyl)hydrazinylidene]propanoic acid Structure](https://image.chemsrc.com/caspic/374/89314-29-4.png) CAS#:89314-29-4
CAS#:89314-29-4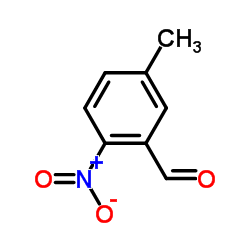 CAS#:5858-28-6
CAS#:5858-28-6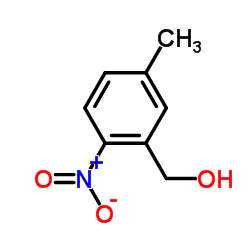 CAS#:66424-92-8
CAS#:66424-92-8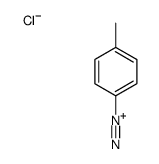 CAS#:2028-84-4
CAS#:2028-84-4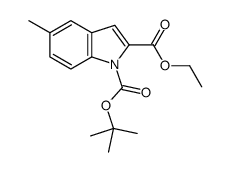 CAS#:1233086-44-6
CAS#:1233086-44-6
