YM-58790 free base
Modify Date: 2025-08-26 23:13:35
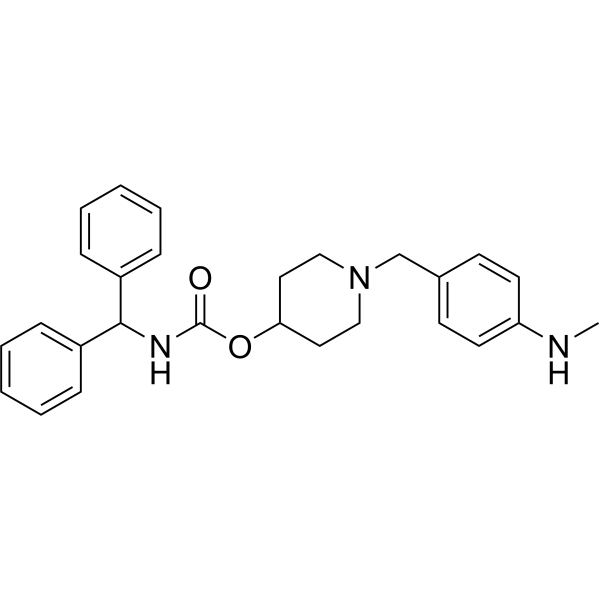
YM-58790 free base structure
|
Common Name | YM-58790 free base | ||
|---|---|---|---|---|
| CAS Number | 168830-70-4 | Molecular Weight | 429.55 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C27H31N3O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of YM-58790 free baseYM-58790 free base is a potent antagonist of mAChR. YM-58790 free base binds M1, M2, M3 with Ki values of 28 nM, 260 nM, and 15 nM. YM-58790 free base exhibits potent inhibitory activity on bladder pressuer in reflexly-evoked rhythmic contraction in rats[1]. |
| Name | YM-58790 free base |
|---|
| Description | YM-58790 free base is a potent antagonist of mAChR. YM-58790 free base binds M1, M2, M3 with Ki values of 28 nM, 260 nM, and 15 nM. YM-58790 free base exhibits potent inhibitory activity on bladder pressuer in reflexly-evoked rhythmic contraction in rats[1]. |
|---|---|
| Related Catalog | |
| Target |
mAChR3:15 nM (Ki) mAChR1:28 nM (Ki) mAChR2:260 nM (Ki) |
| In Vitro | YM-58790 free base (compound 18b) (0-1 μM) shows potent inhibitory effect on urinary bladder contraction, but has little effect on bradycardia. YM-58790 exhibits selective antagonism between urinary bladder contraction and salivary secretion in vitro[1]. |
| In Vivo | YM-58790 free base (3 mg/kg, i.v.) has no effect on oxotremorine-induced tremor in mice[1]. YM-58790 free base (6.0 mg/kg; i.v.) shows poor M1 and M2 antagonism effect in vivo on McN-A343-induced pressor in pithed rats, but displays potent efficacy on M3 antagonism with an ED30 value of 0.36 mg/kg (i.v.) and an ID50 value of 2.4 mg/kg (i.v.)[1]. YM-58790 free base exhibits potent inhibitory activity on bladder pressuer in reflexly-evoked rhythmic contraction, similar to Oxybutynin (HY-B0267), and has about ten times less inhibitory effect on oxotremorine-induced salivary secretion than oxybutynin in rats[1]. |
| Molecular Formula | C27H31N3O2 |
|---|---|
| Molecular Weight | 429.55 |
| InChIKey | YLHJUSKJLHTVJY-UHFFFAOYSA-N |
| SMILES | CNc1ccc(CN2CCC(OC(=O)NC(c3ccccc3)c3ccccc3)CC2)cc1 |