Gold(III) chloride trihydrate
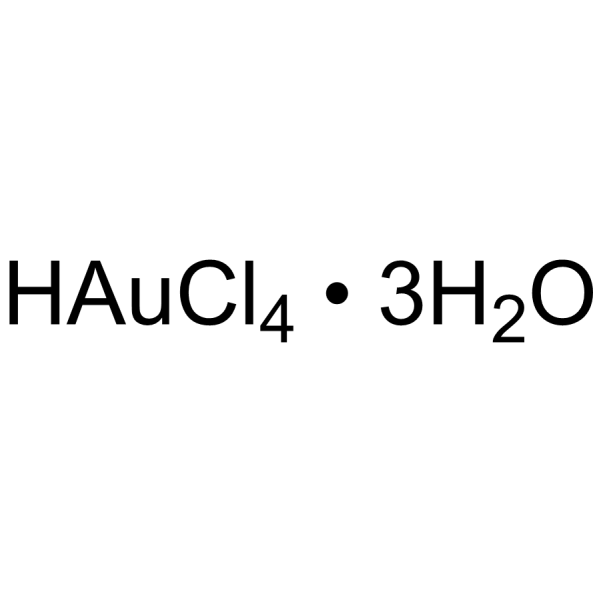
Gold(III) chloride trihydrate structure
|
Common Name | Gold(III) chloride trihydrate | ||
|---|---|---|---|---|
| CAS Number | 16961-25-4 | Molecular Weight | 393.832 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | AuCl4H7O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
Use of Gold(III) chloride trihydrateGold(III) chloride trihydrate is a reducing agent. Gold(III) chloride trihydrate Can be used for the chemical synthesis of gold nanoparticles (NP)[1]. |
| Name | hydrogen tetrachloroaurate(iii) |
|---|---|
| Synonym | More Synonyms |
| Description | Gold(III) chloride trihydrate is a reducing agent. Gold(III) chloride trihydrate Can be used for the chemical synthesis of gold nanoparticles (NP)[1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | AuCl4H7O3 |
|---|---|
| Molecular Weight | 393.832 |
| Exact Mass | 391.881439 |
| PSA | 27.69000 |
| LogP | 2.67510 |
| Storage condition | Refrigerator (+4°C) |
| Stability | Stable, but light sensitive. Very hygroscopic. Incompatible with reducing agents. Protect from moisture and light. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314-H317 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | C |
| Risk Phrases | R34 |
| Safety Phrases | S26-S27-S36/37/39-S45 |
| RIDADR | UN 3260 8/PG 2 |
| WGK Germany | 3 |
| RTECS | MD5428000 |
| Packaging Group | III |
| Hazard Class | 8 |
|
Chitosan-mediated synthesis of gold nanoparticles on patterned poly(dimethylsiloxane) surfaces.
Biomacromolecules 7 (4) , 1203-1209, (2006) Synthesis of gold nanoparticles on surfaces has been accomplished by the incubation of poly(dimethylsiloxane) (PDMS) films in tetrachloroauric(III) acid and chitosan solution at room temperature and 4... |
|
|
Reversible association of thermoresponsive gold nanoparticles: polyelectrolyte effect on the lower critical solution temperature of poly(vinyl methyl ether).
J. Phys. Chem. B 110 (13) , 6768-6775, (2006) Thermoresponsive gold nanoparticles (GNPs) have been prepared by the borohydride reduction of gold salt in the presence of water-soluble polymer, poly(vinyl methyl ether) (PVME). The PVME-coated GNPs ... |
|
|
Nanoglassified, optically-active monolayer films of gold nanoparticles for in situ orthogonal detection by localized surface plasmon resonance and surface-assisted laser desorption/ionization-MS.
Anal. Chem. 86(24) , 11942-5, (2014) Localized surface plasmon resonance (LSPR) represents a sensitive and versatile method for detection of biomolecules in a label-free fashion, but identification of bound analytes can be challenging wi... |
| Aurate(1-), tetrachloro-, hydrogen, hydrate (1:3) |
| GOLD CHLORIDE,HYDRATE |
| AuCl3.3H2O,yellow |
| GOLD (II) CHLORIDE |
| Gold Chloride,Reagent |
| Hydrogen tetrachloroaurate(III) trihydrate |
| EINECS 240-948-4 |
| GOLDCHLORIDE,REAGENT,ACS |
| MFCD00149904 |
| GOLD TRICHLORIDE ACID |
| GOLD CHLORIDE,ACS |
| goldacidchloride |
| Hydrogen tetrachloroaurate(1-) hydrate (1:1:3) |
| Gold(III) chloride trihydrate |

