Strontium Hydroxide
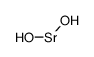
Strontium Hydroxide structure
|
Common Name | Strontium Hydroxide | ||
|---|---|---|---|---|
| CAS Number | 18480-07-4 | Molecular Weight | 121.63500 | |
| Density | 1.47 g/cm3 | Boiling Point | 100ºC at 760 mmHg | |
| Molecular Formula | H2O2Sr | Melting Point | 375ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
| Name | strontium dihydroxide |
|---|---|
| Synonym | More Synonyms |
| Density | 1.47 g/cm3 |
|---|---|
| Boiling Point | 100ºC at 760 mmHg |
| Melting Point | 375ºC |
| Molecular Formula | H2O2Sr |
| Molecular Weight | 121.63500 |
| Exact Mass | 121.91100 |
| PSA | 40.46000 |
| InChIKey | UUCCCPNEFXQJEL-UHFFFAOYSA-L |
| SMILES | [OH-].[OH-].[Sr+2] |
| Stability | Stable. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | C:Corrosive; |
| Risk Phrases | R34 |
| Safety Phrases | S26-S36/37/39-S45 |
| RIDADR | UN 3262 |
| WGK Germany | 3 |
| RTECS | WK9100000 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2816400000 |
| HS Code | 2816400000 |
|---|
|
Enhanced Electroresponse of Alkaline Earth Metal-Doped Silica/Titania Spheres by Synergetic Effect of Dispersion Stability and Dielectric Property.
ACS Appl. Mater. Interfaces 7 , 18977-84, (2015) A series of alkaline earth metal-doped hollow SiO2/TiO2 spheres (EM-HST) are prepared as electrorheological (ER) materials via sonication-mediated etching method with various alkaline earth metal hydr... |
|
|
Syntheses and characterization of Sr(OH)2 and SrCO3 nanostructures by ultrasonic method.
Ultrason. Sonochem. 17(1) , 132-8, (2010) The Sr(OH)(2) and SrCO(3) nanostructures were synthesized by reaction of strontium(II) acetate and sodium hydroxide or tetramethylammonium hydroxide (TMAH) via ultrasonic method. Reaction conditions, ... |
|
|
Ion-pair formation in aqueous strontium chloride and strontium hydroxide solutions under hydrothermal conditions by AC conductivity measurements.
Phys. Chem. Chem. Phys. 16(33) , 17688-704, (2014) Frequency-dependent electrical conductivities of solutions of aqueous strontium hydroxide and strontium chloride have been measured from T = 295 K to T = 625 K at p = 20 MPa, over a very wide range of... |
| strontium dioxide |
| strontium,dihydroxide |
| Strontium dihydroxide |
| MFCD00036273 |
| Strontium hydroxide |
| EINECS 242-367-1 |