Alisol B
Modify Date: 2024-01-08 11:43:40
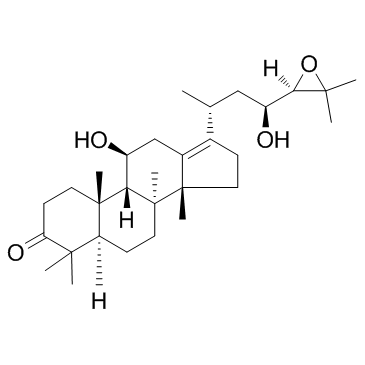
Alisol B structure
|
Common Name | Alisol B | ||
|---|---|---|---|---|
| CAS Number | 18649-93-9 | Molecular Weight | 472.70 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 567.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C30H48O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 181.2±23.6 °C | |
Use of Alisol BAlisol B is a potentially novel therapeutic compound for bone disorders by targeting the differentiation of osteoclasts as well as their functions.IC50 Value:Target:In vitro: The in vitro cultured human renal tubular epithelial HK-2 cells were intervened with 5 ng/mL transforming growth factor-beta (TGF-beta), 0.1 micromol C3a, and 0.1 micromol C3a + 10 micromol alisol B, respectively. Exogenous C3a could induce renal tubular EMT. Alisol B was capable of suppressing C3a induced EMT [1]. Alisol-B strongly inhibited RANKL-induced osteoclast formation when added during the early stage of cultures, suggesting that alisol-B acts on osteoclast precursors to inhibit RANKL/RANK signaling. Among the RANK signaling pathways, alisol-B inhibited the phosphorylation of JNK, which are upregulated in response to RANKL in bone marrow macrophages, alisol-B also inhibited RANKL-induced expression of NFATc1 and c-Fos, which are key transcription factors for osteoclastogenesis. In addition, alisol-B suppressed the pit-forming activity and disrupted the actin ring formation of mature osteoclasts [2]. Alisol B induced calcium mobilization from internal stores, leading to autophagy through the activation of the CaMKK-AMPK-mammalian target of rapamycin pathway. Moreover, the disruption of calcium homeostasis induces endoplasmic reticulum stress and unfolded protein responses in alisol B-treated cells, leading to apoptotic cell death. Finally, by computational virtual docking analysis and biochemical assays, it was showed that the molecular target of alisol B is the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase [3].In vivo: |
| Name | Alisol B |
|---|---|
| Synonym | More Synonyms |
| Description | Alisol B is a potentially novel therapeutic compound for bone disorders by targeting the differentiation of osteoclasts as well as their functions.IC50 Value:Target:In vitro: The in vitro cultured human renal tubular epithelial HK-2 cells were intervened with 5 ng/mL transforming growth factor-beta (TGF-beta), 0.1 micromol C3a, and 0.1 micromol C3a + 10 micromol alisol B, respectively. Exogenous C3a could induce renal tubular EMT. Alisol B was capable of suppressing C3a induced EMT [1]. Alisol-B strongly inhibited RANKL-induced osteoclast formation when added during the early stage of cultures, suggesting that alisol-B acts on osteoclast precursors to inhibit RANKL/RANK signaling. Among the RANK signaling pathways, alisol-B inhibited the phosphorylation of JNK, which are upregulated in response to RANKL in bone marrow macrophages, alisol-B also inhibited RANKL-induced expression of NFATc1 and c-Fos, which are key transcription factors for osteoclastogenesis. In addition, alisol-B suppressed the pit-forming activity and disrupted the actin ring formation of mature osteoclasts [2]. Alisol B induced calcium mobilization from internal stores, leading to autophagy through the activation of the CaMKK-AMPK-mammalian target of rapamycin pathway. Moreover, the disruption of calcium homeostasis induces endoplasmic reticulum stress and unfolded protein responses in alisol B-treated cells, leading to apoptotic cell death. Finally, by computational virtual docking analysis and biochemical assays, it was showed that the molecular target of alisol B is the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase [3].In vivo: |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 567.1±50.0 °C at 760 mmHg |
| Molecular Formula | C30H48O4 |
| Molecular Weight | 472.70 |
| Flash Point | 181.2±23.6 °C |
| PSA | 70.06000 |
| LogP | 4.37 |
| Vapour Pressure | 0.0±3.5 mmHg at 25°C |
| Index of Refraction | 1.560 |
| Storage condition | -20℃ |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| Gon-13(17)-en-3-one, 17-[(1R,3S)-3-[(2S)-3,3-dimethyloxiranyl]-3-hydroxy-1-methylpropyl]-11-hydroxy-8,10,14-trimethyl-, (5α,8α,9β,11β,14β)- |
| (5α,8α,9β,11β,14β,23S,24S)-11,23-Dihydroxy-8,14-dimethyl-24,25-epoxy-18-norcholest-13(17)-en-3-one |
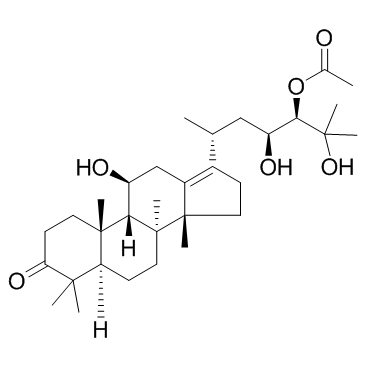 CAS#:18674-16-3
CAS#:18674-16-3
