Cysteine thiol probe
Modify Date: 2025-08-25 08:10:39
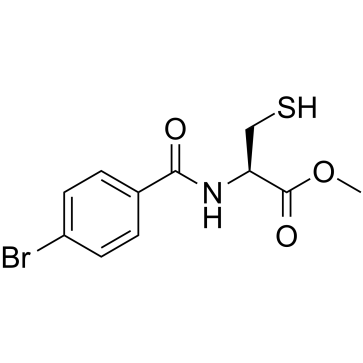
Cysteine thiol probe structure
|
Common Name | Cysteine thiol probe | ||
|---|---|---|---|---|
| CAS Number | 1947408-74-3 | Molecular Weight | 318.19 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C11H12BrNO3S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Cysteine thiol probeCysteine Thiol Probe is a thiol-based probe designed to label electrophilic natural products. Cysteine Thiol Probe possesses each of the characteristics of an ideal pharmacophore probe, and has a chromophore. Cysteine Thiol Probe is capable of engaging enone-, β-lactam-, and β-lactone-based electrophilic metabolites[1][2]. |
| Name | Cysteine thiol probe |
|---|
| Description | Cysteine Thiol Probe is a thiol-based probe designed to label electrophilic natural products. Cysteine Thiol Probe possesses each of the characteristics of an ideal pharmacophore probe, and has a chromophore. Cysteine Thiol Probe is capable of engaging enone-, β-lactam-, and β-lactone-based electrophilic metabolites[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Cysteine Thiol Probe (Probe 1) reacts much more readily with β-lactam- and β-lactone-based electrophilic natural products, and its reactivity with epoxide-based electrophilic natural products is poor. And probe 2 reacts much faster with epoxide-containing natural products. This orthogonal reactivity allows them to be employed in extracts simultaneously. Since Cysteine Thiol Probe is brominated and probe 2 is chlorinated, the isotopic patterns of labeled natural products point toward their structural origin. Competition experiments using Cysteine Thiol Probe and 2 indicated that brominated Cysteine Thiol Probe reacted exclusively with the β-lactam in penicillin G and the β-lactone in salinosporamide A while chlorinated probe 2 alone reacted with the epoxide in salinamide A[1]. |
| References |
| Molecular Formula | C11H12BrNO3S |
|---|---|
| Molecular Weight | 318.19 |
| InChIKey | QLAHQHTYCQKQLI-VIFPVBQESA-N |
| SMILES | COC(=O)C(CS)NC(=O)c1ccc(Br)cc1 |
| Storage condition | 2-8°C |