Fusicoccin-A
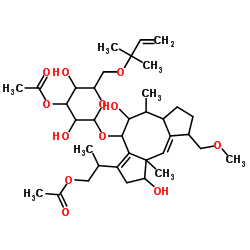
Fusicoccin-A structure
|
Common Name | Fusicoccin-A | ||
|---|---|---|---|---|
| CAS Number | 20108-30-9 | Molecular Weight | 680.823 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 760.2±60.0 °C at 760 mmHg | |
| Molecular Formula | C36H56O12 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 227.0±26.4 °C | |
Use of Fusicoccin-AFusicoccin (Fusicoccin A), a fungal pytotoxin, is a stabilizer of specific 14-3-3 protein-protein interactions. Fusicoccin sabilizes H+-ATPase/14-3-3 cmplex in pants, maintaining the enzyme in activated state. Fusicoccin also stabilizes 14-3-3 protein interactions with binding partners containing a C-terminal 14-3-3 recognition motif (a mode 3 motif), such as ERα, GPIbα, TASK3, CTFR, and p53. Fusicoccin induces apoptosis in cancer cells and has anticancer activity[1][2][3][4]. |
| Name | fusicoccin |
|---|---|
| Synonym | More Synonyms |
| Description | Fusicoccin (Fusicoccin A), a fungal pytotoxin, is a stabilizer of specific 14-3-3 protein-protein interactions. Fusicoccin sabilizes H+-ATPase/14-3-3 cmplex in pants, maintaining the enzyme in activated state. Fusicoccin also stabilizes 14-3-3 protein interactions with binding partners containing a C-terminal 14-3-3 recognition motif (a mode 3 motif), such as ERα, GPIbα, TASK3, CTFR, and p53. Fusicoccin induces apoptosis in cancer cells and has anticancer activity[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Fusicoccin (Fusicoccin A) stabilizes a complex between 14-3-3 and the stress response regulator GCN1, inducing GCN1 turnover and neurite outgrowth (EC50=29 mM)[3]. Fusicoccin A activates the plasma membrane H+-ATPase by stabilizing its binding to 14-3-3 proteins, which results in water loss and the wilting of infected plants. Fusicoccin A decreases the proliferation and migration of human GBM cell lines in vitro, including several cell lines that exhibit varying degrees of resistance to pro-apoptotic stimuli. The IC50 growth inhibitory concentration of fusicoccin A is 92 µM in the U373-MG cells and 83 µM in the Hs683 glioma cells[4]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 760.2±60.0 °C at 760 mmHg |
| Molecular Formula | C36H56O12 |
| Molecular Weight | 680.823 |
| Flash Point | 227.0±26.4 °C |
| Exact Mass | 680.377197 |
| PSA | 170.44000 |
| LogP | 3.28 |
| Vapour Pressure | 0.0±5.8 mmHg at 25°C |
| Index of Refraction | 1.555 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Identification and characterisation of the phenolics of Ilex glabra L. Gray (Aquifoliaceae) leaves by liquid chromatography tandem mass spectrometry.
Plant Physiol. Biochem. 70 , 504-11, (2013) The phenolics of the leaves of Ilex glabra L. Gray (Aquifoliaceae) were investigated qualitatively by LC-MS(n). Thirty-two phenolics were detected and characterised on the basis of their unique fragme... |
|
|
Apoplastic alkalinization is instrumental for the inhibition of cell elongation in the Arabidopsis root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid.
Plant Physiol. 155(4) , 2049-55, (2011) In Arabidopsis (Arabidopsis thaliana; Columbia-0) roots, the so-called zone of cell elongation comprises two clearly different domains: the transition zone, a postmeristematic region (approximately 20... |
|
|
Characterization of the plasma membrane H+-ATPase in the liverwort Marchantia polymorpha.
Plant Physiol. 159(2) , 826-34, (2012) The plasma membrane H(+)-ATPase generates an electrochemical gradient of H(+) across the plasma membrane that provides the driving force for solute transport and regulates pH homeostasis and membrane ... |
| 2-[(9aE)-4-{[3-O-Acetyl-6-O-(2-methyl-3-buten-2-yl)hexopyranosyl]oxy}-1,5-dihydroxy-9-(methoxymethyl)-6,10a-dimethyl-1,2,4,5,6,6a,7,8,9,10a-decahydrodicyclopenta[a,d][8]annulen-3-yl]propyl acetate |
| MFCD00070322 |
| FUSICOCCIN |
| 3-[2-(Acetyloxy)-1-methylethyl]-1,2,4,5,6,6a,7,8,9,10a-decahydro-1,5-dihydroxy-9-(methoxymethyl)-6,10a-dimethylcyclopenta[a,d]-dicylo coten-4-yl-6 |
| Hexopyranoside, (9aE)-3-[2-(acetyloxy)-1-methylethyl]-1,2,4,5,6,6a,7,8,9,10a-decahydro-1,5-dihydroxy-9-(methoxymethyl)-6,10a-dimethyldicyclopenta[a,d]cycloocten-4-yl 6-O-(1,1-dimethyl-2-propen-1-yl)-, 3-acetate |
| Fucicoccin |
| fusicoccin A |