AMI 1
Modify Date: 2024-01-09 21:17:36
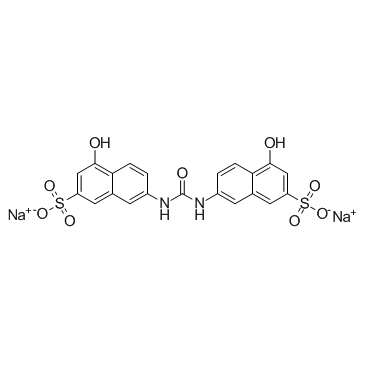
AMI 1 structure
|
Common Name | AMI 1 | ||
|---|---|---|---|---|
| CAS Number | 20324-87-2 | Molecular Weight | 548.453 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C21H14N2Na2O9S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of AMI 1AMI-1 is a potent, cell-permeable compound which inhibits protein arginine N-methyltransferases (PRMTs), including human PRMT1 (IC50 = 8.8μM) and yeast-Hmt1p (IC50 = 3.0μM), by blocking peptide-substrate binding.IC50 value: 8.8μM (human PRMT1), 3.0μM (yeast-Hmt1p)Target: human PRMT1, yeast-Hmt1pin vitro: AMI-1 suppresses the transcriptional coactivator activity of PRMT1 and PRMT4 and it inhibits HIV-1 RT polymerase (IC50 = 5.0μM). PRMT1 methylates histone H4, and is essential for other subsequent histone modifications.[1] AMI-1 is the most active nonpeptidic inhibitor reported to be selective against PRMT1. AMI-1 is a selective PRMT inhibitor with a bisanionic structure that is related to compounds known to generate pleiotropic interactions with many proteins, should be further optimized before exploring additional binding pockets. [2]in vivo: AMI-1 is administered intranasally to chronic AIPI rats to determine PRMT effects on asthmatic parameters. AMI-1 inhibited the expression of COX2 in TGF-β-stimulated cells. AMI-1 administered to AIPI rats reduced COX2 production and humoral immune response, and it abrogated mucus secretion and collagen generation.[1] |
| Name | Disodium 7,7'-(carbonyldiimino)bis(4-hydroxynaphthalene-2-sulphonate) |
|---|---|
| Synonym | More Synonyms |
| Description | AMI-1 is a potent, cell-permeable compound which inhibits protein arginine N-methyltransferases (PRMTs), including human PRMT1 (IC50 = 8.8μM) and yeast-Hmt1p (IC50 = 3.0μM), by blocking peptide-substrate binding.IC50 value: 8.8μM (human PRMT1), 3.0μM (yeast-Hmt1p)Target: human PRMT1, yeast-Hmt1pin vitro: AMI-1 suppresses the transcriptional coactivator activity of PRMT1 and PRMT4 and it inhibits HIV-1 RT polymerase (IC50 = 5.0μM). PRMT1 methylates histone H4, and is essential for other subsequent histone modifications.[1] AMI-1 is the most active nonpeptidic inhibitor reported to be selective against PRMT1. AMI-1 is a selective PRMT inhibitor with a bisanionic structure that is related to compounds known to generate pleiotropic interactions with many proteins, should be further optimized before exploring additional binding pockets. [2]in vivo: AMI-1 is administered intranasally to chronic AIPI rats to determine PRMT effects on asthmatic parameters. AMI-1 inhibited the expression of COX2 in TGF-β-stimulated cells. AMI-1 administered to AIPI rats reduced COX2 production and humoral immune response, and it abrogated mucus secretion and collagen generation.[1] |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C21H14N2Na2O9S2 |
|---|---|
| Molecular Weight | 548.453 |
| Exact Mass | 547.993591 |
| PSA | 212.75000 |
| LogP | 5.16400 |
| Storage condition | -20℃ |
| HS Code | 2924299090 |
|---|
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
| 2-Naphthalenesulfonic acid, 7,7'-(carbonyldiimino)bis[4-hydroxy-, sodium salt (1:2) |
| Disodium 4-hydroxy-7-{[(5-hydroxy-7-sulfonato-2-naphthyl)carbamoyl]amino}naphthalene-2-sulfonate |
| Disodium 7,7'-(carbonyldiimino)bis(4-hydroxy-2-naphthalenesulfonate) |
| AMI-1 |