Luteollin 5-glucoside
Modify Date: 2025-08-25 18:55:45
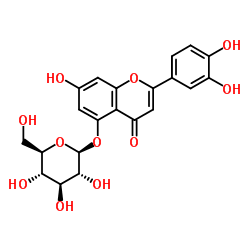
Luteollin 5-glucoside structure
|
Common Name | Luteollin 5-glucoside | ||
|---|---|---|---|---|
| CAS Number | 20344-46-1 | Molecular Weight | 448.377 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 864.2±65.0 °C at 760 mmHg | |
| Molecular Formula | C21H20O11 | Melting Point | 260-263℃ | |
| MSDS | N/A | Flash Point | 305.8±27.8 °C | |
Use of Luteollin 5-glucosideLuteolin 5-O-glucoside, a major flavonoidfrom Cirsium maackii, possesses anti-inflammatory activity. Luteolin 5-O-glucoside inhibits LPS-induced NO production and t-BHP-induced ROS generation. Luteolin 5-O-glucoside suppresses the expression of iNOS and COX-2 in macrophages[1]. |
| Name | 2-(3,4-dihydroxyphenyl)-7-hydroxy-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one |
|---|---|
| Synonym | More Synonyms |
| Description | Luteolin 5-O-glucoside, a major flavonoidfrom Cirsium maackii, possesses anti-inflammatory activity. Luteolin 5-O-glucoside inhibits LPS-induced NO production and t-BHP-induced ROS generation. Luteolin 5-O-glucoside suppresses the expression of iNOS and COX-2 in macrophages[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Luteolin 5-O-glucoside, at a non-toxic concentration, inhibits LPS-induced NO production and t-BHP-induced ROS generation in a dose-dependent manner in RAW 264.7 cells. Luteolin 5-O-glucoside also suppresses the expression of iNOS and COX-2 in LPS-stimulated macrophages[1]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 864.2±65.0 °C at 760 mmHg |
| Melting Point | 260-263℃ |
| Molecular Formula | C21H20O11 |
| Molecular Weight | 448.377 |
| Flash Point | 305.8±27.8 °C |
| Exact Mass | 448.100555 |
| PSA | 190.28000 |
| LogP | 0.13 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.740 |
| InChIKey | KBGKQZVCLWKUDQ-QNDFHXLGSA-N |
| SMILES | O=c1cc(-c2ccc(O)c(O)c2)oc2cc(O)cc(OC3OC(CO)C(O)C(O)C3O)c12 |
| HS Code | 2932999099 |
|---|
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| Luteolin-5-O-b-D-glucopyranoside |
| 2-(3,4-Dihydroxyphenyl)-5-(b-D-glucopyranosyloxy)-7-hydroxy-4H-1-benzopyran-4-one |
| Luteolin-5-O-glucopyranoside |
| 2-(3,4-Dihydroxyphenyl)-7-hydroxy-4-oxo-4H-chromen-5-yl β-D-glucopyranoside |
| Luteolin-5-O-β-D-glucopyranoside |
| Luteollin 5-glucoside |
| 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-5-(β-D-glucopyranosyloxy)-7-hydroxy- |
| galuteolin |
| luteolin 5-O-β-D-glucopyranosyl |
 CAS#:491-70-3
CAS#:491-70-3
