ethyl 1,3-dithiolane-2-carboxylate
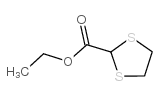
ethyl 1,3-dithiolane-2-carboxylate structure
|
Common Name | ethyl 1,3-dithiolane-2-carboxylate | ||
|---|---|---|---|---|
| CAS Number | 20461-99-8 | Molecular Weight | 178.27200 | |
| Density | 1.249 g/mL at 25 °C(lit.) | Boiling Point | 85 °C0.1 mm Hg(lit.) | |
| Molecular Formula | C6H10O2S2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | >230 °F | |
| Name | ethyl 1,3-dithiolane-2-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.249 g/mL at 25 °C(lit.) |
|---|---|
| Boiling Point | 85 °C0.1 mm Hg(lit.) |
| Molecular Formula | C6H10O2S2 |
| Molecular Weight | 178.27200 |
| Flash Point | >230 °F |
| Exact Mass | 178.01200 |
| PSA | 76.90000 |
| LogP | 1.35560 |
| Vapour Pressure | 0.013mmHg at 25°C |
| Index of Refraction | n20/D 1.539(lit.) |
| InChIKey | OMCSHTHLIQOHDD-UHFFFAOYSA-N |
| SMILES | CCOC(=O)C1SCCS1 |
| Personal Protective Equipment | Eyeshields;Gloves |
|---|---|
| Safety Phrases | S23-S24/25 |
| RIDADR | 3334 |
| WGK Germany | 3 |
| HS Code | 2934999090 |
|
~% 
ethyl 1,3-dithi... CAS#:20461-99-8 |
| Literature: Journal of the Indian Chemical Society, , vol. 5, p. 457 Chem. Zentralbl., , vol. 99, # II p. 2254 |
|
~% 
ethyl 1,3-dithi... CAS#:20461-99-8 |
| Literature: Journal of the Indian Chemical Society, , vol. 5, p. 546 Chem. Zentralbl., , vol. 99, # II p. 2253 Journal of the Indian Institute of Science, Section A, vol. 11, p. 228 Chem. Zentralbl., , vol. 100, # I p. 1697 |
|
~79% 
ethyl 1,3-dithi... CAS#:20461-99-8 |
| Literature: Lissel, Manfred Liebigs Annalen der Chemie, 1982 , # 9 p. 1589 - 1601 |
|
~% 
ethyl 1,3-dithi... CAS#:20461-99-8 |
| Literature: Journal of the Indian Institute of Science, , vol. 11, p. 227,229 Chem. Zentralbl., , vol. 99, # II p. 2253 |
| Precursor 4 | |
|---|---|
| DownStream 5 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
General strategy for the syntheses of corynanthe, tacaman, and oxindole alkaloids.
J. Org. Chem. 71(17) , 6547-61, (2006) We report herein the total synthesis of the corynanthe alkaloid dihydrocorynantheol and the formal syntheses of the indole alkaloids tacamonine, rhynchophylline, and hirsutine. The strategies for asse... |
|
|
Stereodivergent synthesis of enantiopure cis- and trans-3-Ethyl-4-piperidineacetates.
Org. Lett. 4(16) , 2787-90, (2002) [reaction: see text] Starting from a common chiral bicyclic lactam 11, enantiopure trans- or cis-3-ethyl-4-piperidineacetate derivatives are obtained by conjugate addition of an enolate or a cuprate t... |
|
|
H. Paulson, W. Koebernick
Chem. Ber. 110 , 2127, (1977)
|
| 2-Carboethoxydithiolane |
| 1,3-Dithiolane-2-carboxylic Acid Ethyl Ester |
| Ethyl-1,3-dithiolan-2-carboxylat |
| Ethyl 1,3-dithiolane-2-carboxylate |
| ethyl dithiolane-2-carboxylate |
| 2-ethoxycarbonyl-1,3-dithiolane |
| ethyl 2,2-(1,3-dithiolane-2-yl)acetate |
| MFCD00005411 |
| EINECS 243-837-9 |


 CAS#:182417-47-6
CAS#:182417-47-6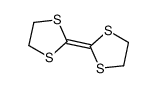 CAS#:24719-68-4
CAS#:24719-68-4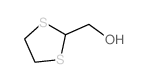 CAS#:86032-47-5
CAS#:86032-47-5![7-acetyl-6-methoxy-9-oxa-1,4-dithiaspiro[4.5]decan-10-one structure](https://image.chemsrc.com/caspic/248/89665-14-5.png) CAS#:89665-14-5
CAS#:89665-14-5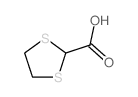 CAS#:5616-65-9
CAS#:5616-65-9