Sodium Aescinate
Modify Date: 2025-08-22 14:25:45
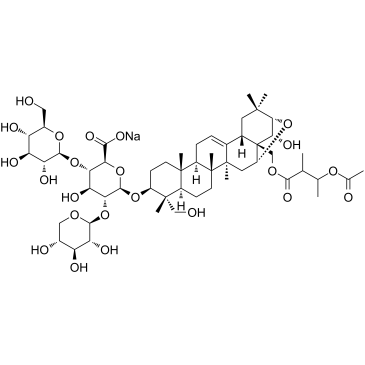
Sodium Aescinate structure
|
Common Name | Sodium Aescinate | ||
|---|---|---|---|---|
| CAS Number | 20977-05-3 | Molecular Weight | 1124.2202 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C54H83NaO23 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Sodium AescinateSodium aescinate is a triterpene saponin derived from Aesculus hippocastanum seeds, with anti-inflammatory and antioxidant activities[1]. Sodium aescinate inhibits hepatocellular carcinoma growth by targeting CARMA3/NF-κB pathway[2]. |
| Name | Escin monosodium salt |
|---|---|
| Synonym | More Synonyms |
| Description | Sodium aescinate is a triterpene saponin derived from Aesculus hippocastanum seeds, with anti-inflammatory and antioxidant activities[1]. Sodium aescinate inhibits hepatocellular carcinoma growth by targeting CARMA3/NF-κB pathway[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Sodium aescinate can block signals transiting to downstream molecules AKT, ERK, inhibit the proliferation of breast cancer cell MCF-7 cell apoptosis and induced cell apoptosis by suppressing the activation of SRC[3]. |
| In Vivo | Sodium aescinate may effectively controls and improves wound healing in diabetic rats via its anti-inflammatory and antioxidant activities[1]. Sodium aescinate treatment can alleviate the symptom of polycystic ovary syndrome (PCOS) in rat model through regulating the PI3K/Akt/GSK3-β pathway[4]. |
| References |
| Molecular Formula | C54H83NaO23 |
|---|---|
| Molecular Weight | 1124.2202 |
| InChIKey | OJTQULAMLNBGOY-NREQLCGPSA-N |
| SMILES | CC(=O)OC(C)C(C)C(=O)OCC12C3CC4(C)C(=CCC5C6(C)CCC(OC7OC(C(=O)O)C(OC8OC(CO)C(O)C(O)C8O)C(O)C7OC7OCC(O)C(O)C7O)C(C)(CO)C6CCC54C)C1CC(C)(C)C(O3)C2O.[Na+] |
| 3-methyl-pyridine-2-carbonitrile |
| 3-methyl-2-pyridinecarbonitrile |
| 2-cyano-3-methly pyridine |
| 2-cyano-b-picoline |
| 3-methylpyridine-2-carbonitrile |
| 2-Cyano-3-methylpyridine |
| (h4tes4)[qr] |
| akrochem tdec |
| diethyl-carbamodithioicacitetrakis(anhydrosulfide)withthiotelluricac |
| Sodium escinate |

