2'-Iodoacetophenone
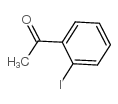
2'-Iodoacetophenone structure
|
Common Name | 2'-Iodoacetophenone | ||
|---|---|---|---|---|
| CAS Number | 2142-70-3 | Molecular Weight | 246.04500 | |
| Density | 1.72 g/mL at 25 °C(lit.) | Boiling Point | 139-140°C 12mm | |
| Molecular Formula | C8H7IO | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 218 °F | |
| Name | 1-(2-iodophenyl)ethanone |
|---|---|
| Synonym | More Synonyms |
| Density | 1.72 g/mL at 25 °C(lit.) |
|---|---|
| Boiling Point | 139-140°C 12mm |
| Molecular Formula | C8H7IO |
| Molecular Weight | 246.04500 |
| Flash Point | 218 °F |
| Exact Mass | 245.95400 |
| PSA | 17.07000 |
| LogP | 2.49380 |
| Vapour Pressure | 0.00779mmHg at 25°C |
| Index of Refraction | n20/D 1.618(lit.) |
| InChIKey | XDXCBCXNCQGZPG-UHFFFAOYSA-N |
| SMILES | CC(=O)c1ccccc1I |
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| Hazard Codes | C: Corrosive; |
| Risk Phrases | R36/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914700090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |
|
Trapping evidence for the thermal cyclization of di-(o-acetylphenyl)acetylene to 3,3'-dimethyl-1,1'-biisobenzofuran.
Beilstein J. Org. Chem. 1(1) , 18, (2005) The reaction of di-(o-acetylphenyl)acetylene (1) with excess dimethyl acetylenedicarboxylate (DMAD) produced bis-DMAD adducts meso-3 and rac-3. This transformation is suggested to involve thermal rear... |
|
|
Cobalt-catalyzed regioselective carbocyclization reaction of o-iodophenyl ketones and aldehydes with alkynes, acrylates, and acrylonitrile: a facile route to indenols and indenes.
J. Org. Chem. 69(14) , 4781-7, (2004) An efficient cobalt-catalyzed carbocylization for the synthesis of indenols and indenes and a new method for reductive decyanation are described. 2-Iodophenyl ketones and aldehydes 1a-g undergo carboc... |
|
|
Cobalt-Catalyzed Carbocyclization of o-Iodobenzaldehydes and o-Iodophenylketones with Alkynes. Chang KJ, et al.
Org. Lett. 5(21) , 3963-3966, (2003)
|
| 1-(2-iodophenyl)ethan-1-one |
| 2-IC6H4COMe |
| 2′-Iodoacetophenone |
| MFCD00094998 |
| 2-Ac-C6H4-I |
| 2‘-Iodoacetophenone |
| o-Iodoacetophenone |
| ortho-iodoacetophenone |
| 2'-Iodoacetophenone |
| 2-Iodoacetophenone |
| 2'-IODO-1,1':3',1''-TERPHENYL |
| o-acetyl-iodobenzene |
| 1-acetyl-2-iodobenzene |
| Acetophenone,o-iodo |
| 1-(2-Iodophenyl)ethanone |
 CAS#:551-93-9
CAS#:551-93-9 CAS#:615-42-9
CAS#:615-42-9 CAS#:108-24-7
CAS#:108-24-7 CAS#:98-86-2
CAS#:98-86-2 CAS#:122752-70-9
CAS#:122752-70-9 CAS#:69352-04-1
CAS#:69352-04-1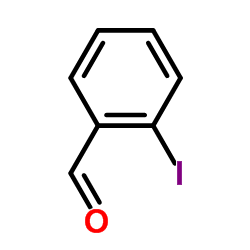 CAS#:26260-02-6
CAS#:26260-02-6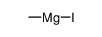 CAS#:917-64-6
CAS#:917-64-6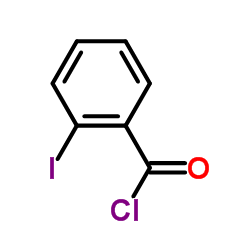 CAS#:609-67-6
CAS#:609-67-6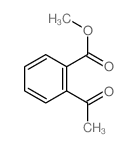 CAS#:1077-79-8
CAS#:1077-79-8 CAS#:3609-53-8
CAS#:3609-53-8 CAS#:20731-95-7
CAS#:20731-95-7![[N-(trifluoromethanesulfonyl)imino][2-(acetyl)phenyl]-λ3-iodane structure](https://image.chemsrc.com/caspic/050/1133467-80-7.png) CAS#:1133467-80-7
CAS#:1133467-80-7 CAS#:402-43-7
CAS#:402-43-7 CAS#:3176-62-3
CAS#:3176-62-3 CAS#:611-64-3
CAS#:611-64-3 CAS#:118-93-4
CAS#:118-93-4
