6-AMINO-5-BROMOPYRIMIDIN-2(1H)-ONE
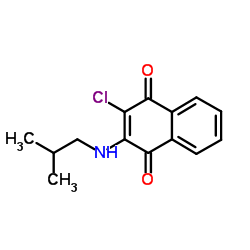
6-AMINO-5-BROMOPYRIMIDIN-2(1H)-ONE structure
|
Common Name | 6-AMINO-5-BROMOPYRIMIDIN-2(1H)-ONE | ||
|---|---|---|---|---|
| CAS Number | 2240-25-7 | Molecular Weight | 190.00 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 359.5±42.0 °C at 760 mmHg | |
| Molecular Formula | C4H4BrN3O | Melting Point | 240-243ºC | |
| MSDS | USA | Flash Point | 171.2±27.9 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
Use of 6-AMINO-5-BROMOPYRIMIDIN-2(1H)-ONE4-Amino-5-bromopyrimidin-2(1H)-one is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 6-amino-5-bromo-1H-pyrimidin-2-one |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Amino-5-bromopyrimidin-2(1H)-one is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 359.5±42.0 °C at 760 mmHg |
| Melting Point | 240-243ºC |
| Molecular Formula | C4H4BrN3O |
| Molecular Weight | 190.00 |
| Flash Point | 171.2±27.9 °C |
| Exact Mass | 263.071320 |
| PSA | 72.03000 |
| LogP | 3.14 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.583 |
| Water Solubility | Soluble in formic acid (50 mg/ml). |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H317-H318-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Hazard Codes | Xn |
| Risk Phrases | 22-37/38-41-43-48-20/21/22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933599090 |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Structure-activity relationships of beta-D-(2S,5R)- and alpha-D-(2S,5S)-1,3-oxathiolanyl nucleosides as potential anti-HIV agents.
J. Med. Chem. 36(18) , 2627-38, (1993) The beta-D-(2S,5R)- and alpha-D-(2S,5S)-1,3-oxathiolanylpyrimidine and -purine nucleosides with natural nucleoside configuration were synthesized and evaluated against HIV-1 in human peripheral blood ... |
|
|
Formation of intrastrand cross-link products between cytosine and adenine from UV irradiation of d((Br)CA) and duplex DNA containing a 5-bromocytosine.
J. Am. Chem. Soc. 127(40) , 13969-77, (2005) Here, we showed that Pyrex-filtered UV light irradiation of d((Br)CA) gave rise to three types of intrastrand cross-link products, that is, d(C[5-N6]A), d(C[5-2]A), and d(C[5-8]A), where the C5 carbon... |
|
|
Lifetime regulation of the charge-separated state in DNA by modulating the oxidation potential of guanine in DNA through hydrogen bonding.
J. Am. Chem. Soc. 126(40) , 12843-6, (2004) A series of naphthalimide (NI)- and 5-bromocytosine ((br)C)-modified oligodeoxynucleotides (ODNs) were prepared, and their lifetimes of the charge-separated states during the photosensitized one-elect... |
| EINECS 218-806-8 |
| 1,4-Naphthalenedione, 2-chloro-3-[(2-methylpropyl)amino]- |
| 5-bromoycytosine |
| 4-Amino-5-bromo-2-hydroxypyrimidine |
| 2-chloro-3-(isobutylamino)-1,4-dihydronaphthalene-1,4-dione |
| MFCD00056025 |
| 4-amino-5-bromopyrimidin-2-ol |
| 5-Bromocytosine |
| bromocytosine |
| 4-amino-5-bromo-1H-pyrimidin-2-one |
| 6-AMINO-5-BROMOPYRIMIDIN-2(1H)-ONE |
| 2-Chloro-3-(isobutylamino)-1,4-naphthoquinone |
| F3096-1966 |

