Hyponine E
Modify Date: 2025-08-25 13:38:33
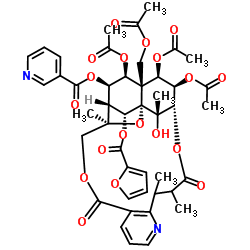
Hyponine E structure
|
Common Name | Hyponine E | ||
|---|---|---|---|---|
| CAS Number | 226975-99-1 | Molecular Weight | 920.865 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 913.2±65.0 °C at 760 mmHg | |
| Molecular Formula | C45H48N2O19 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 506.1±34.3 °C | |
Use of Hyponine EHyponine E, a macrocyclic sesquiterpene pyridine alkaloid that could be isolated from from Tripterygium hypoglaucum, possesses anti-inflammatory effects[1][2]. |
| Name | Hyponine E |
|---|---|
| Synonym | More Synonyms |
| Description | Hyponine E, a macrocyclic sesquiterpene pyridine alkaloid that could be isolated from from Tripterygium hypoglaucum, possesses anti-inflammatory effects[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 913.2±65.0 °C at 760 mmHg |
| Molecular Formula | C45H48N2O19 |
| Molecular Weight | 920.865 |
| Flash Point | 506.1±34.3 °C |
| Exact Mass | 920.285156 |
| LogP | 5.73 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.603 |
| InChIKey | PMRSIAJYXABCTQ-ZQDMINLRSA-N |
| SMILES | CC(=O)OCC12C(OC(C)=O)C(OC(=O)c3cccnc3)C3C(OC(=O)c4ccco4)C14OC3(C)COC(=O)c1cccnc1C(C)C(C)C(=O)OC(C(OC(C)=O)C2OC(C)=O)C4(C)O |
| Hyponine E |
| (1S,3R,17S,18R,19R,20R,21S,22R,23R,24R,25S)-18,19,21-Triacetoxy-20-(acetoxymethyl)-24-(2-furoyloxy)-25-hydroxy-3,13,14,25-tetramethyl-6,15-dioxo-2,5,16-trioxa-11-azapentacyclo[15.7.1.0.0.0 ]pentacosa-7,9,11-trien-22-yl nicotinate |