Benzo[h]quinoline
![Benzo[h]quinoline Structure](https://image.chemsrc.com/caspic/349/230-27-3.png)
Benzo[h]quinoline structure
|
Common Name | Benzo[h]quinoline | ||
|---|---|---|---|---|
| CAS Number | 230-27-3 | Molecular Weight | 179.217 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 340.8±0.0 °C at 760 mmHg | |
| Molecular Formula | C13H9N | Melting Point | 48-50 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 155.9±11.9 °C | |
| Name | benzo[h]quinoline |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 340.8±0.0 °C at 760 mmHg |
| Melting Point | 48-50 °C(lit.) |
| Molecular Formula | C13H9N |
| Molecular Weight | 179.217 |
| Flash Point | 155.9±11.9 °C |
| Exact Mass | 179.073502 |
| PSA | 12.89000 |
| LogP | 3.32 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.727 |
| InChIKey | WZJYKHNJTSNBHV-UHFFFAOYSA-N |
| SMILES | c1ccc2c(c1)ccc1cccnc12 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DK1432000 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 8 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis of novel benzo[h]quinolines: Wound healing, antibacterial, DNA binding and in vitro antioxidant activity
Eur. J. Med. Chem. 44 , 981-9, (2009) We have characterized a new class of 2-mercapto/2-selenobenzo[ h]quinoline-3-carbaldehyde ( 3/ 4). Antibacterial potential of these compounds against a wide range of Gram-positive and Gram-negative ba... |
|
|
Determination of basic nitrogen-containing polynuclear aromatic hydrocarbons formed during thermal degradation of polymers by high-performance liquid chromatography-fluorescence detection.
J. Chromatogr. A. 878(2) , 171-81, (2000) A method for the simultaneous determination of 22 nitrogen-containing polynuclear aromatic hydrocarbons (PAHs) (15 azaarenes and seven amino-PAHs) in the gaseous products of the thermal degradation of... |
|
|
Mutagenicity and tumorigenicity of dihydrodiols, diol epoxides, and other derivatives of benzo(f)quinoline and benzo(h)quinoline.
Cancer Res. 49(1) , 20-4, (1989) The mutagenic activities of benzo[f]quinoline, benzo[h]quinoline, and a number of their derivatives, including dihydrodiols, K-region oxides, diol epoxides, and tetrahydroepoxides, were assessed in st... |
| 7,8-Bnzoquinoline |
| α-Naphthoquinoline |
| Benzo[h]quinoline |
| 5-AZAPHENANTHRENE |
| benzoquinoline |
| 7,8-Benzoquinoline |
| benzo<h>quinoline |
| EINECS 205-937-0 |
| 7,8-Benoz(h)quinoline |
| MFCD00004984 |
| 4-Azaphenanthrene |
| BENZO(H)QUINOLINE |
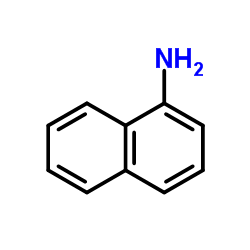 CAS#:134-32-7
CAS#:134-32-7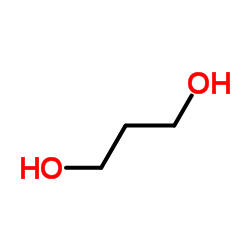 CAS#:504-63-2
CAS#:504-63-2![2-bromobenzo[h]quinoline Structure](https://image.chemsrc.com/caspic/152/1097204-18-6.png) CAS#:1097204-18-6
CAS#:1097204-18-6![4-chlorobenzo[h]quinoline Structure](https://image.chemsrc.com/caspic/417/181950-47-0.png) CAS#:181950-47-0
CAS#:181950-47-0![2-chlorobenzo[h]quinoline Structure](https://image.chemsrc.com/caspic/336/202523-63-5.png) CAS#:202523-63-5
CAS#:202523-63-5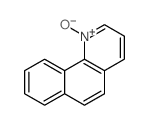 CAS#:17104-70-0
CAS#:17104-70-0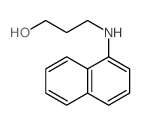 CAS#:6628-35-9
CAS#:6628-35-9 CAS#:64-17-5
CAS#:64-17-5 CAS#:201230-82-2
CAS#:201230-82-2![9-nitrobenzo[h]quinoline structure](https://image.chemsrc.com/caspic/243/186268-22-4.png) CAS#:186268-22-4
CAS#:186268-22-4![Quinolino[8,7-h]quinoline structure](https://image.chemsrc.com/caspic/108/218-34-8.png) CAS#:218-34-8
CAS#:218-34-8![5H-Indeno[1,2-b]pyridine structure](https://image.chemsrc.com/caspic/095/244-99-5.png) CAS#:244-99-5
CAS#:244-99-5![1H-benzo[h]quinolin-10-one structure](https://image.chemsrc.com/caspic/181/33155-90-7.png) CAS#:33155-90-7
CAS#:33155-90-7![10-acetoxybenzo[h]quinoline structure](https://image.chemsrc.com/caspic/359/83491-10-5.png) CAS#:83491-10-5
CAS#:83491-10-5![5H-indeno[1,2-b]pyridin-5-ol structure](https://image.chemsrc.com/caspic/242/127664-00-0.png) CAS#:127664-00-0
CAS#:127664-00-0
