1H-Benz[g]indole
Modify Date: 2025-08-21 09:07:55
![1H-Benz[g]indole Structure](https://image.chemsrc.com/caspic/208/233-34-1.png)
1H-Benz[g]indole structure
|
Common Name | 1H-Benz[g]indole | ||
|---|---|---|---|---|
| CAS Number | 233-34-1 | Molecular Weight | 167.20700 | |
| Density | 1.229g/cm3 | Boiling Point | 371.1ºC at 760mmHg | |
| Molecular Formula | C12H9N | Melting Point | 180-184ºC | |
| MSDS | Chinese USA | Flash Point | 168.8ºC | |
| Symbol |


GHS05, GHS06 |
Signal Word | Danger | |
| Name | 1H-Benz[g]indole 6,7-Benzindole NSC 153687 |
|---|---|
| Synonym | More Synonyms |
| Density | 1.229g/cm3 |
|---|---|
| Boiling Point | 371.1ºC at 760mmHg |
| Melting Point | 180-184ºC |
| Molecular Formula | C12H9N |
| Molecular Weight | 167.20700 |
| Flash Point | 168.8ºC |
| Exact Mass | 167.07300 |
| PSA | 15.79000 |
| LogP | 3.32110 |
| Vapour Pressure | 2.26E-05mmHg at 25°C |
| Index of Refraction | 1.767 |
| InChIKey | HIYWOHBEPVGIQN-UHFFFAOYSA-N |
| SMILES | c1ccc2c(c1)ccc1cc[nH]c12 |
| Symbol |


GHS05, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H318 |
| Precautionary Statements | P280-P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | 22-41 |
| Safety Phrases | 26 |
| RIDADR | UN 2811 6.1/PG 3 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Microwave assisted Leimgruber-Batcho reaction for the preparation of indoles, azaindoles and pyrroylquinolines.
Org. Biomol. Chem. 2 , 160, (2004) The development of enhanced conditions for Lewis acid catalysed Leimgruber-Batcho indole synthesis using microwave acceleration is described. This approach has permitted the preparation of a variety o... |
| 1h-benzo(g)indole |
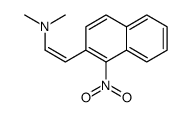 CAS#:176853-40-0
CAS#:176853-40-0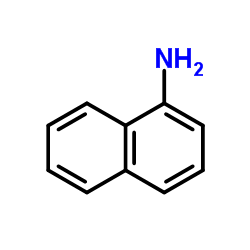 CAS#:134-32-7
CAS#:134-32-7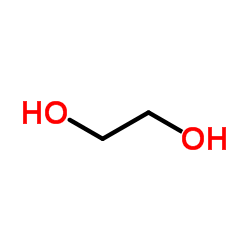 CAS#:107-21-1
CAS#:107-21-1![4,5-dihydro-1H-benzo[g]indole Structure](https://image.chemsrc.com/caspic/116/4995-14-6.png) CAS#:4995-14-6
CAS#:4995-14-6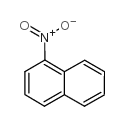 CAS#:86-57-7
CAS#:86-57-7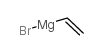 CAS#:1826-67-1
CAS#:1826-67-1 CAS#:881-03-8
CAS#:881-03-8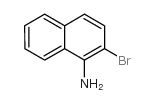 CAS#:771-14-2
CAS#:771-14-2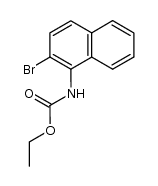 CAS#:107784-16-7
CAS#:107784-16-7![1H-Benzo[g]indole-3-carboxaldehyde structure](https://image.chemsrc.com/caspic/449/51136-18-6.png) CAS#:51136-18-6
CAS#:51136-18-6