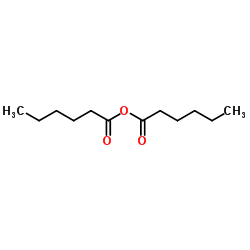
N-caproylglycine
Modify Date: 2025-08-24 11:20:06

N-caproylglycine structure
|
Common Name | N-caproylglycine | ||
|---|---|---|---|---|
| CAS Number | 24003-67-6 | Molecular Weight | 173.210 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 387.2±25.0 °C at 760 mmHg | |
| Molecular Formula | C8H15NO3 | Melting Point | 90-92°C | |
| MSDS | N/A | Flash Point | 188.0±23.2 °C | |
Use of N-caproylglycineHexanoylglycine is an endogenous metabolite present in Urine that can be used for the research of Ethylmalonic Encephalopathy[1][2]. |
| Name | N-hexanoylglycine |
|---|---|
| Synonym | More Synonyms |
| Description | Hexanoylglycine is an endogenous metabolite present in Urine that can be used for the research of Ethylmalonic Encephalopathy[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Endogenous metabolites is defined as those that are annotated by Kyoto Encyclopedia of Genes and Genomes as substrates or products of the ~1900 metabolic enzymes encoded in our genome. It is clear in the body of literature that there are documented toxic properties for many of these metabolites[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 387.2±25.0 °C at 760 mmHg |
| Melting Point | 90-92°C |
| Molecular Formula | C8H15NO3 |
| Molecular Weight | 173.210 |
| Flash Point | 188.0±23.2 °C |
| Exact Mass | 173.105194 |
| PSA | 66.40000 |
| LogP | 0.71 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.462 |
| InChIKey | UPCKIPHSXMXJOX-UHFFFAOYSA-N |
| SMILES | CCCCCC(=O)NCC(=O)O |
| Storage condition | Refrigerator |
| HS Code | 2924199090 |
|---|
| Precursor 8 | |
|---|---|
| DownStream 2 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
| Hexanoyl Glycine |
| Acide (hexanoylamino)acétique |
| 2-(hexanoylamino)acetic acid |
| N-Hexanoylglycine |
| N-hexanoyl-Glycine |
| N-caproylglycine |
| (Hexanoylamino)acetic acid |
| Glycine, N-(1-oxohexyl)- |

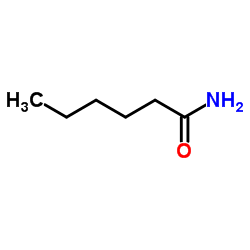

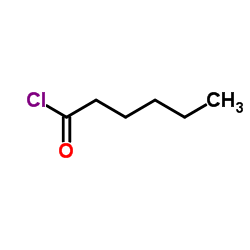
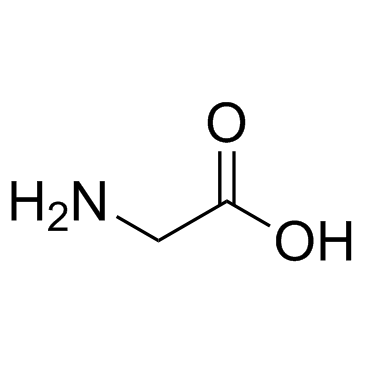
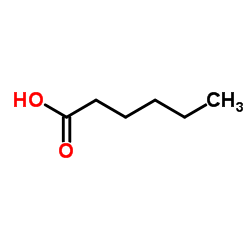

 CAS#:881-90-3
CAS#:881-90-3 CAS#:31295-09-7
CAS#:31295-09-7