Clobetasol
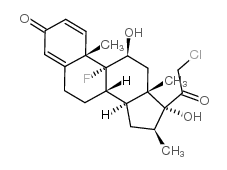
Clobetasol structure
|
Common Name | Clobetasol | ||
|---|---|---|---|---|
| CAS Number | 25122-41-2 | Molecular Weight | 410.90700 | |
| Density | 1.32 g/cm3 | Boiling Point | 555.1ºC at 760 mmHg | |
| Molecular Formula | C22H28ClFO4 | Melting Point | 195 - 197C (decomposes)ºC | |
| MSDS | Chinese USA | Flash Point | 289.5ºC | |
| Symbol |

GHS08 |
Signal Word | Danger | |
| Name | clobetasol |
|---|---|
| Synonym | More Synonyms |
| Density | 1.32 g/cm3 |
|---|---|
| Boiling Point | 555.1ºC at 760 mmHg |
| Melting Point | 195 - 197C (decomposes)ºC |
| Molecular Formula | C22H28ClFO4 |
| Molecular Weight | 410.90700 |
| Flash Point | 289.5ºC |
| Exact Mass | 410.16600 |
| PSA | 74.60000 |
| LogP | 3.14220 |
| Vapour Pressure | 1.23E-14mmHg at 25°C |
| Index of Refraction | 1.582 |
| InChIKey | FCSHDIVRCWTZOX-DVTGEIKXSA-N |
| SMILES | CC1CC2C3CCC4=CC(=O)C=CC4(C)C3(F)C(O)CC2(C)C1(O)C(=O)CCl |
| Storage condition | -20?C Freezer |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H360Df-H373-H413 |
| Precautionary Statements | P201-P308 + P313 |
| Target Organs | Adrenal gland, Immune system |
| Hazard Codes | T |
| Risk Phrases | 61-48/20/21-53-62 |
| Safety Phrases | 53-36/37-45 |
| RIDADR | NONH for all modes of transport |
|
Topical steroids for chronic wounds displaying abnormal inflammation.
Ann. R. Coll. Surg. Engl. 95(4) , 291-6, (2013) Chronic, non-healing wounds are often characterised by an excessive, and detrimental, inflammatory response. We review our experience of using a combined topical steroid, antibiotic and antifungal pre... |
|
|
Impact of clobetasol propionate 0.05% spray on health-related quality of life in patients with plaque psoriasis.
Journal. of. Drugs in. Dermatology. 11(11) , 1348-54, (2012) Psoriasis causes significant distress and impairment in health-related quality of life (QOL) in afflicted patients. For this reason, QOL is an essential and important measure of treatment outcome in p... |
|
|
Clobetasol propionate spray 0.05% for the treatment of moderate to severe plaque psoriasis.
Cutis. 89(2) , 89-94, (2012) Clobetasol propionate is a super-high potent class 1 topical corticosteroid available in several formulations, including a spray formulation that is approved for use up to 4 weeks in patients aged 18 ... |
| (8S,9R,10S,11S,13S,14S,16S,17R)-17-(2-chloroacetyl)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one |
| UNII-ADN79D536H |
| Clobetasolum |
| EINECS 246-633-8 |
| Clobetasol [INN:BAN] |
| 21-Chloro-9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione |
| Clobetasolum [INN-Latin] |