Mitotane-d8
Modify Date: 2025-09-02 10:55:08
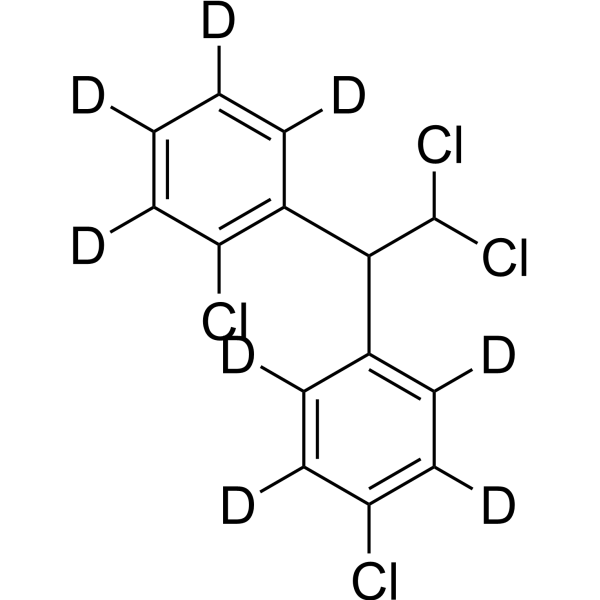
Mitotane-d8 structure
|
Common Name | Mitotane-d8 | ||
|---|---|---|---|---|
| CAS Number | 2673270-14-7 | Molecular Weight | 328.09 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C14H2D8Cl4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Mitotane-d8Mitotane-d8 is the deuterium labeled Mitotane[1]. Mitotane (2,4′-DDD), an isomer of DDD and derivative of dichlorodiphenyltrichloroethane (DDT), is an antineoplastic agent, can be used to research adrenocortical carcinoma. Mitotane exert its adrenocorticolytic effect at least in part through lipotoxicity induced by intracellular free cholesterol (FC) accumulation. Mitotane can have direct pituitary effects on corticotroph cells. Mitotane can induce CYP3A4 gene expression via steroid and xenobiotic receptor (SXR) activation, and has drug-drug interactions[2][3][4][5]. |
| Name | Mitotane-d8 |
|---|
| Description | Mitotane-d8 is the deuterium labeled Mitotane[1]. Mitotane (2,4′-DDD), an isomer of DDD and derivative of dichlorodiphenyltrichloroethane (DDT), is an antineoplastic agent, can be used to research adrenocortical carcinoma. Mitotane exert its adrenocorticolytic effect at least in part through lipotoxicity induced by intracellular free cholesterol (FC) accumulation. Mitotane can have direct pituitary effects on corticotroph cells. Mitotane can induce CYP3A4 gene expression via steroid and xenobiotic receptor (SXR) activation, and has drug-drug interactions[2][3][4][5]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C14H2D8Cl4 |
|---|---|
| Molecular Weight | 328.09 |