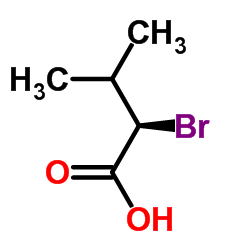
(S)-(-)-2-Bromo-3-methylbutyric acid
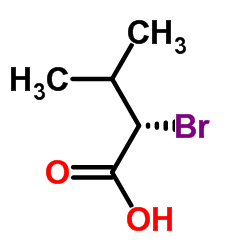
(S)-(-)-2-Bromo-3-methylbutyric acid structure
|
Common Name | (S)-(-)-2-Bromo-3-methylbutyric acid | ||
|---|---|---|---|---|
| CAS Number | 26782-75-2 | Molecular Weight | 181.028 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 230.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C5H9BrO2 | Melting Point | 39-44ºC | |
| MSDS | USA | Flash Point | 107.2±0.0 °C | |
| Symbol |

GHS05, GHS07 |
Signal Word | Danger | |
| Name | (2S)-2-bromo-3-methylbutanoic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 230.0±0.0 °C at 760 mmHg |
| Melting Point | 39-44ºC |
| Molecular Formula | C5H9BrO2 |
| Molecular Weight | 181.028 |
| Flash Point | 107.2±0.0 °C |
| Exact Mass | 179.978592 |
| PSA | 37.30000 |
| LogP | 1.73 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.487 |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H312-H314-H332 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | C: Corrosive; |
| Risk Phrases | R20/21/22;R34 |
| Safety Phrases | S26-S27-S28-S36/37/39-S45 |
| RIDADR | UN 3261 |
| HS Code | 2915900090 |
| HS Code | 2915900090 |
|---|---|
| Summary | 2915900090 other saturated acyclic monocarboxylic acids and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:5.5% General tariff:30.0% |
|
Glutathione conjugation of the alpha-bromoisovaleric acid enantiomers in the rat in vivo and its stereoselectivity. Pharmacokinetics of biliary and urinary excretion of the glutathione conjugate and the mercapturate.
Biochem. Pharmacol. 38(22) , 3957-62, (1989) The glutathione (GSH) conjugation of (R)-and (S)-alpha-bromoisovaleric acid (BI) in the rat in vivo, and its stereoselectivity, have been characterized. After administration of racemic [1-14C]BI two r... |
|
|
Stereoselectivity of rat liver glutathione transferase isoenzymes for alpha-bromoisovaleric acid and alpha-bromoisovalerylurea enantiomers.
Biochem. J. 252(1) , 137-42, (1988) The stereoselectivity of purified rat GSH transferases towards alpha-bromoisovaleric acid (BI) and its amide derivative alpha-bromoisovalerylurea (BIU) was investigated. GSH transferase 2-2 was the on... |
|
|
Stereoselective conjugation of 2-bromocarboxylic acids and their urea derivatives by rat liver glutathione transferase 12-12 and some other isoforms.
Biochem. Pharmacol. 44(7) , 1249-53, (1992) Glutathione (GSH) conjugation of the separate enantiomers of five 2-bromocarboxylic acids and some of their urea derivatives by rat liver GSH transferases (GSTs) was studied. The liver cytosolic fract... |
| MFCD00210114 |
| α-BROMOISOVALERIC ACID, (S)- |
| (2S)-2-bromo-3-methyl-butanoic acid |
| 2-Bromo-3-methylbutyric acid |
| Butanoic acid, 2-bromo-3-methyl- |
| 2S-2-bromo-3-methylbutanoic acid |
| (2S)-2-Bromo-3-methylbutanoic acid |
| α-Bromoisovaleric acid |
| (±)-2-BROMO-3-METHYLBUTYRIC ACID |
| (S)-2-Bromoisovaleric acid |
| Isovaleric acid, α-bromo- |
| 2-Bromo-3-methylbutanoic acid |
| (S)-2-Bromo-3-methylbutyric acid |
| Butanoic acid, 2-bromo-3-methyl-, (2S)- |
| (S)-(-)-2-Bromo-3-methylbutyric acid |
| S-2-Bromo-3-methyl-butyric acid |
| UNII:M69LGV465C |
![1-[1,2,5,6-di-O-(1-methylethylidene)-α-D-glucofuranosyloxy]-1-trimethylsilyloxy-3-methyl-but-1-ene structure](https://www.chemsrc.com/caspic/341/161661-13-8.png)
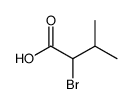
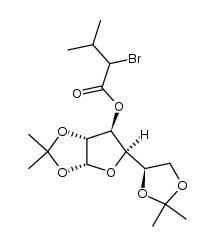
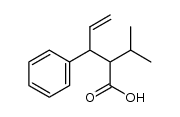 CAS#:1310051-05-8
CAS#:1310051-05-8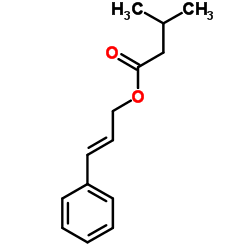 CAS#:140-27-2
CAS#:140-27-2