| In Vitro |
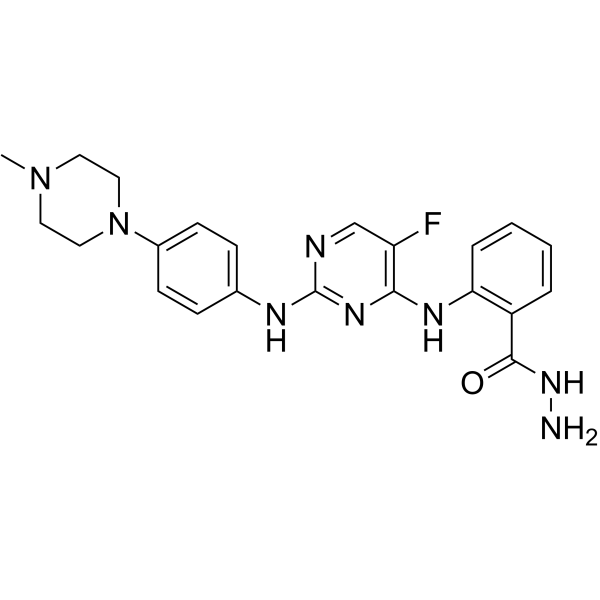
RSH-7 (1-1000 nM; 72 h) 显示出抗增殖活性,对 Jeko-1、MV-4-11、Molt4、K562 细胞的 IC50 值分别为 17、3、11、930 nM[1]。 RSH-7 (30, 150, 750 nM; 72 h) 以剂量依赖性方式降低 p-BTK (TYR223)、p-PLCγ(Tyr1217)、p-FLT3 (Tyr589)、p-STAT5 (TYR694) 的表达[1]。 RSH-7 (30, 150, 750 nM; 72 h) 在 jeko-1 细胞中以剂量依赖性方式诱导细胞凋亡并增加 BAX、p53、cleaved caspase 3 蛋白的表达[1]。 Cell Viability Assay[1] Cell Line: Jeko-1, MV-4-11, Molt4, K562 cells Concentration: 1-1000 nM Incubation Time: 72 h Result: Showed antiproliferative activities with IC50s of 17, 3, 11, 930 nM for Jeko-1, MV-4-11, Molt4, K562 cells, respectively. Western Blot Analysis[1] Cell Line: jeko-1 cells Concentration: 30, 150, 750 nM Incubation Time: 72 h Result: Reduced both BTK, PLCγ2, FLT3 and STAT5 phosphorylation in a dose-dependent manner. Apoptosis Analysis[1] Cell Line: jeko-1 cells Concentration: 30, 150, 750 nM Incubation Time: 72 h Result: Dose-dependently induced cell apoptosis and upregulated the expression of pro-apoptotic protein BAX, p53, cleaved caspase 3.
|
| In Vivo |
RSH-7 (25、50 mg/kg;腹腔注射;每日一次,持续 16 天) 显示出抗肿瘤活性,剂量依赖性地抑制小鼠的肿瘤生长[1]。 Animal Model: Female NOD/SCID mice (jeko-1 cell-inoculated xenograft NOD/SCID mice models)[1] Dosage: 25, 50 mg/kg Administration: I.p.; daily for 16 days Result: Suppressed tumor growth in a dose-dependent manner, with tumor growth inhibition (TGI) values of 66.95% and 79.78% at doses of 25 and 50 mg/kg, Animal Model: Female NOD/SCID mice (MV4-11 cell-inoculated xenograft NOD/SCID mice models)[1] Dosage: 10, 20 mg/kg Administration: I.p.; daily for 21 days Result: Significantly and dose-dependently suppressed the tumor growth with the TGI rates of 74.23% and 94.84% at the dosage of 10 and 20 mg/kg, respectively.
|