Tetrahydropalmatine
Modify Date: 2025-08-26 22:33:26
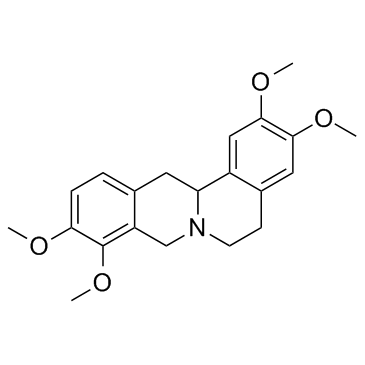
Tetrahydropalmatine structure
|
Common Name | Tetrahydropalmatine | ||
|---|---|---|---|---|
| CAS Number | 2934-97-6 | Molecular Weight | 355.427 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 482.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C21H25NO4 | Melting Point | 155℃ | |
| MSDS | N/A | Flash Point | 138.7±25.9 °C | |
Use of TetrahydropalmatineTetrahydropalmatine, an active component isolated from corydalis, acts through inhibition of amygdaloid release of dopamine to inhibit an epileptic attack in rats. |
| Name | rotundinum |
|---|---|
| Synonym | More Synonyms |
| Description | Tetrahydropalmatine, an active component isolated from corydalis, acts through inhibition of amygdaloid release of dopamine to inhibit an epileptic attack in rats. |
|---|---|
| Related Catalog | |
| Target |
Dopamine[1] |
| In Vivo | Tetrahydropalmatine (THP), an active component isolated from corydalis (a Chinese herbal medicine), possesses analgesic effects. Picrotoxin treatment alone has a significant effect on the following activity measure: there is an increase in horizontal motion time (HMT), vertical motion time (VMT), clockwise turnings (CT), anticlockwise turning (ACT) and a decrease in freezing time (FT). Tetrahydropalmatine treatment alone causes a decrease in HMT, VMT and total distance traveled (TDT), but an increase in FT. Pretreatment of rats with an i.p. dose of 10 mg/kg or 15 mg/kg of Tetrahydropalmatine significantly attenuates the Picrotoxin-induced enhancement in HMT, VMT, CT, ACT and TDT, as well as reduction in FT. Another 48 rats under urethane anesthesia are randomly divided into six groups, each of eight rats. The s.c. injection of Picrotoxin causes an increase in amygdaloid release of dopamine (DA), while i.p. injection of Tetrahydropalmatine at 10 mg/kg has an insignificant effect on amygdaloid release of DA. Again, the Picrotoxin-induced increase in amygdaloid release of DA is significantly attenuated by pretreatment with Tetrahydropalmatine. The Picrotoxin-induced augmented amygdaloid release of DA is almost completely abolished by pretreatment with Tetrahydropalmatine 30 min before s.c. injection of Picrotoxin[1]. |
| Animal Admin | Rats[1] Male Sprague-Dawley rats, weighing 250-320 g, are used in the present study. The animals are housed in a temperature-regulated (22±1°C) room on 12:12 h light/dark cycles with food and water available ad libitum. The light is turned on at 06:00 h and turned off at 18:00 h. At least two major groups of animals are studied. (1) Vehicle-treated rats: received an i.p. injection of 0.9% saline plus Picrotoxin (3-4 mg/kg, s.c.). (2) Tetrahydropalmatine-treated rats: receive an injection of Tetrahydropalmatine (10 mg/kg, i.p.) plus Picrotoxin (3-4 mg/kg, s.c.). The effects of s.c. administration of Picrotoxin or Tetrahydropalmatine on locomotor activity are assessed in unanesthetized rats. On the other hand, the effects of Picrotoxin or Tetrahydropalmatine on amygdaloid DA release are assessed in rats under urethane (1.4 g/kg, i.p.) anesthesia[1]. Seventy-two unanesthetized rats are randomly divided into nine groups, each of eight rats. The animals are adapted to the behavior apparatus for 30 min before an injection of Picrotoxin (3 or 4 mg/kg, s.c.), Tetrahydropalmatine (10 or 15 mg/kg, i.p.) or saline. Then, the locomotor activities of these rats are recorded during the 30-min period following the injections[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 482.9±45.0 °C at 760 mmHg |
| Melting Point | 155℃ |
| Molecular Formula | C21H25NO4 |
| Molecular Weight | 355.427 |
| Flash Point | 138.7±25.9 °C |
| Exact Mass | 355.178345 |
| PSA | 40.16000 |
| LogP | 3.70 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.609 |
| HS Code | 2923900090 |
|---|
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 6H-Dibenzo[a,g]quinolizine, 5,8,13,13a-tetrahydro-2,3,9,10-tetramethoxy-, (13aS)- |
| Berberine,tetrahydro |
| d,l-tetrahydropalmatine |
| (S)-tetrahydropalmatine |
| (13aS)-2,3,9,10-Tetramethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinoline |
| Tetrahydropalmatine |
| (13aS)-2,3,9,10-Tetramethoxy-5,8,13,13a-tetrahydro-6H-isoquino[3,2-a]isoquinoline |
| dl-Canadine |
| l-Tetrahydropalmatine |
| (-)-Tetrahydropalmatine |
| 2,3-methylenedioxy-9,10-dimethoxytetrahydroprotoberberine |
| Canadine dl-form |
| Canadin |
| tetrahydropulmatine |
![5,6,13,13a-tetrahydro-2,3,9,10-tetramethoxy-8H-dibenzo[a,g]quinolizin-8-one Structure](https://image.chemsrc.com/caspic/320/81701-50-0.png) CAS#:81701-50-0
CAS#:81701-50-0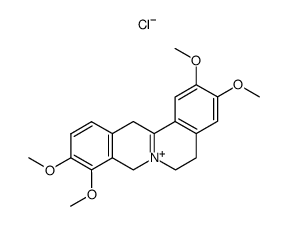 CAS#:75875-59-1
CAS#:75875-59-1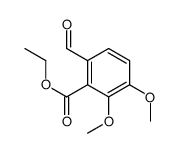 CAS#:104270-87-3
CAS#:104270-87-3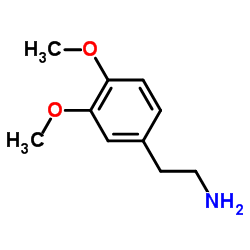 CAS#:120-20-7
CAS#:120-20-7 CAS#:86639-32-9
CAS#:86639-32-9 CAS#:86-51-1
CAS#:86-51-1 CAS#:483-14-7
CAS#:483-14-7![2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium,iodide structure](https://image.chemsrc.com/caspic/399/4880-79-9.png) CAS#:4880-79-9
CAS#:4880-79-9
