3520-14-7
| Name | D-Tetrahydropalmatine |
|---|---|
| Synonyms |
levo-tetrahydropalmatine
(S)-tetrahydropalmatine (13aS)-2,3,9,10-Tetramethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinoline Tetrahydropalmatine (+)-Tetrahydropalmatine (13aS)-2,3,9,10-Tetramethoxy-5,8,13,13a-tetrahydro-6H-isoquino[3,2-a]isoquinoline d-Tetrahydropalmatine l-Tetrahydropalmatine (-)-Tetrahydropalmatine (R)-(+)-tetrahydropalmatine D-Tetrahydropalmatin (13aR)-2,3,9,10-Tetramethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinoline |
| Description | D-Tetrahydropalmatine is an isoquinoline alkaloid, mainly in the genus Corydalis[1]. D-Tetrahydropalmatine is a Dopamine (DA) receptor antagonist with preferential affinity toward the D1 receptors[2]. D-Tetrahydropalmatine is a potent organic cation transporter 1 (OCT1) inhibitor[3]. |
|---|---|
| Related Catalog | |
| Target |
DA[2], OCT1[3] |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 482.9±45.0 °C at 760 mmHg |
| Molecular Formula | C21H25NO4 |
| Molecular Weight | 355.427 |
| Flash Point | 138.7±25.9 °C |
| Exact Mass | 355.178345 |
| PSA | 40.16000 |
| LogP | 3.70 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.609 |
| Hazard Codes | Xi |
|---|
|
~% 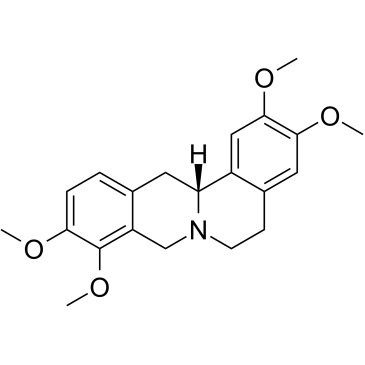
3520-14-7 |
| Literature: Journal of Organic Chemistry, , vol. 70, # 23 p. 9486 - 9494 |
| Precursor 1 | |
|---|---|
| DownStream 2 | |



![2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium,iodide structure](https://image.chemsrc.com/caspic/399/4880-79-9.png)