Ethyl (4-cyanophenyl)(oxo)acetate
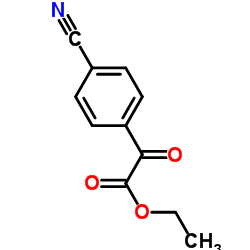
Ethyl (4-cyanophenyl)(oxo)acetate structure
|
Common Name | Ethyl (4-cyanophenyl)(oxo)acetate | ||
|---|---|---|---|---|
| CAS Number | 302912-31-8 | Molecular Weight | 203.194 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 350.3±25.0 °C at 760 mmHg | |
| Molecular Formula | C11H9NO3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 153.0±13.4 °C | |
| Name | ethyl 2-(4-cyanophenyl)-2-oxoacetate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 350.3±25.0 °C at 760 mmHg |
| Molecular Formula | C11H9NO3 |
| Molecular Weight | 203.194 |
| Flash Point | 153.0±13.4 °C |
| Exact Mass | 203.058243 |
| PSA | 67.16000 |
| LogP | 1.45 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.536 |
| InChIKey | MGBZXMQPYAYPIQ-UHFFFAOYSA-N |
| SMILES | CCOC(=O)C(=O)c1ccc(C#N)cc1 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| HS Code | 2926909090 |
|
~55% 
Ethyl (4-cyanop... CAS#:302912-31-8 |
| Literature: Tada, Norihiro; Ban, Kazunori; Nobuta, Tomoya; Hirashima, Shin-Ichi; Miura, Tsuyoshi; Itoh, Akichika Synlett, 2011 , # 10 art. no. U01411ST, p. 1381 - 1384 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| HS Code | 2926909090 |
|---|---|
| Summary | HS:2926909090 other nitrile-function compounds VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Harnessing Candida tenuis and Pichia stipitis in whole-cell bioreductions of o-chloroacetophenone: stereoselectivity, cell activity, in situ substrate supply and product removal.
Biotechnol. J. 8(6) , 699-708, (2013) Generally, recombinant and native microorganisms can be employed as whole-cell catalysts. The application of native hosts, however, shortens the process development time by avoiding multiple steps of ... |
|
|
Whole-cell bioreduction of aromatic alpha-keto esters using Candida tenuis xylose reductase and Candida boidinii formate dehydrogenase co-expressed in Escherichia coli.
Microb. Cell Fact. 7 , 37, (2008) Whole cell-catalyzed biotransformation is a clear process option for the production of chiral alcohols via enantioselective reduction of precursor ketones. A wide variety of synthetically useful reduc... |
| MFCD01319614 |
| ethyl 2-(4-cyanophenyl)-2-oxo-acetate |
| Ethyl (4-cyanophenyl)(oxo)acetate |
| (4-cyano-phenyl)-oxo-acetic acid ethyl ester |
| Ethyl 2-(4-cyanophenyl)-2-oxoacetate |
| ethyl (4-cyanophenyl)glyoxylate |
| Ethyl 4-cyanobenzoylformate |
| Benzeneacetic acid, 4-cyano-α-oxo-, ethyl ester |
| ethyl (4-cyanophenyl)-oxo-acetate |

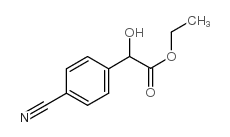 CAS#:847227-46-7
CAS#:847227-46-7
