AGK2
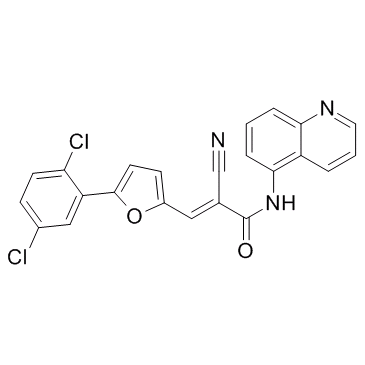
AGK2 structure
|
Common Name | AGK2 | ||
|---|---|---|---|---|
| CAS Number | 304896-28-4 | Molecular Weight | 434.274 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 675.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C23H13Cl2N3O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 362.1±31.5 °C | |
Use of AGK2AGK2 is a selective SIRT2 inhibitor with IC50 of 3.5 μM. AGK2 can also inhibit SIRT1 and SIRT3 with IC50 of 30 and 91 μM, respectively. |
| Name | 2-Cyano-3-[[5-(2,5-dichlorophenyl)-2-furanyl]-N-5-quinolinyl-2-propenamide |
|---|---|
| Synonym | More Synonyms |
| Description | AGK2 is a selective SIRT2 inhibitor with IC50 of 3.5 μM. AGK2 can also inhibit SIRT1 and SIRT3 with IC50 of 30 and 91 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
SIRT2:3.5 μM (IC50) SIRT1:30 μM (IC50) SIRT3:91 μM (IC50) |
| In Vitro | AGK2 significantly inhibits cell proliferation in a dose-dependent manner. AGK2 also significantly inhibits cell growth in a dose-dependent manner without inducing cytotoxicity at low doses. Twelve days after AGK2 (5 μM) treatment, cells show a significantly reducing colony forming ability in soft agar to 46% of the control cells. Western blot analysis shows that the levels of CDK4 or CDK6 and cyclin D1 are decreased after AGK2 treatment in a dose-dependent manner. In addition, AGK2 inhibits the expression of p53 protein[2]. Treatment of microglial BV2 cells with 10 μM AGK2 leads to a significant increase in PAR signals. Treatment of microglial BV2 cells with 10 μM AGK2 also leads to a significant decrease in the intracellular ATP and significant increases in both late-stage apoptosis and necrosis of the cells[3]. |
| In Vivo | AGK2 significantly reduces mortality and decreases levels of cytokines in blood (TNF-α: 298.3±24.6 vs 26.8±2.8 pg/mL, p=0.0034; IL-6: 633.4±82.8 vs 232.6±133.0 pg/mL, p=0.0344) and peritoneal fluid (IL-6: 704.8±67.7 vs 391.4±98.5 pg/mL, p=0.033) compare to vehicle control. AGK2 also suppresses the TNF-α and IL-6 production in the culturing splenocytes (TNF-α: 68.1±6.4 vs 23.9±2.8 pg/mL, p=0.0009; IL-6: 73.1±4.2 vs 49.6±3.0 pg/mL; p=0.0051)[4]. |
| Cell Assay | Cells are exposed to different concentrations of AGK2 in 1 mL of 0.3% basal medium agar containing 10% FBS. The cultures are maintained at 37°C in a 5% CO2 incubator for 10-15 days, and the cell colonies are scored using an inverted microscope[2]. |
| Animal Admin | Mice are intraperitoneally given either AGK2 (82 mg/kg) in dimethyl sulfoxide (DMSO) or DMSO alone, and 2 h later subjects to CLP. Survival is monitored for 240 hours. AGK2-treating mice are grouped into (i) DMSO vehicle, and (ii) AGK2, with sham mice (operating but without any treatment) serving as controls. Peritoneal fluid and peripheral blood are examined at 24 and 48 hours for cytokine production[4]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 675.1±55.0 °C at 760 mmHg |
| Molecular Formula | C23H13Cl2N3O2 |
| Molecular Weight | 434.274 |
| Flash Point | 362.1±31.5 °C |
| Exact Mass | 433.038483 |
| PSA | 78.92000 |
| LogP | 5.39 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.718 |
| InChIKey | SVENPFFEMUOOGK-SDNWHVSQSA-N |
| SMILES | N#CC(=Cc1ccc(-c2cc(Cl)ccc2Cl)o1)C(=O)Nc1cccc2ncccc12 |
| Storage condition | Room temp |
| Water Solubility | DMSO: soluble2mg/mL, clear (warmed) |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36 |
| Safety Phrases | 26 |
| RIDADR | NONH for all modes of transport |
|
Functionalized tetrahydro-1H-pyrido[4,3-b]indoles: a novel chemotype with Sirtuin 2 inhibitory activity.
Eur. J. Med. Chem. 92 , 145-55, (2015) Sirtuins are protein deacylases with regulatory roles in metabolism and stress response. Functionalized tetrahydro-1H-pyrido[4,3-b]indoles were identified as preferential sirtuin 2 inhibitors, with in... |
|
|
SIRT2 ameliorates lipopolysaccharide-induced inflammation in macrophages.
Biochem. Biophys. Res. Commun. 450(4) , 1363-9, (2014) SIRT2 is a NAD(+)-dependent deacetylases and associated with numerous processes such as infection, carcinogenesis, DNA damage and cell cycle regulation. However, the role of SIRT2 in inflammatory proc... |
|
|
Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging.
EMBO J. 33(12) , 1321-40, (2014) Neural stem/progenitor cell (NSPC) proliferation and self-renewal, as well as insult-induced differentiation, decrease markedly with age. The molecular mechanisms responsible for these declines remain... |
| ARC 239 dihydrochloride |
| (2E)-2-cyano-3-[5-(2,5-dichlorophenyl)furan-2-yl]-N-(quinolin-5-yl)prop-2-enamide |
| (2E)-2-Cyano-3-[5-(2,5-dichlorophenyl)-2-furyl]-N-(5-quinolinyl)acrylamide |
| AGK2 |
| 2-Propenamide, 2-cyano-3-[5-(2,5-dichlorophenyl)-2-furanyl]-N-5-quinolinyl-, (2E)- |
| (2E)-2-Cyano-3-[5-(2,5-dichlorophenyl)-2-furyl]-N-(quinolin-5-yl)acrylamide |
| 2-Cyano-3-[5-(2,5-dichlorophenyl)-2-furanyl]-N-5-quinolinyl-2-propenamide |