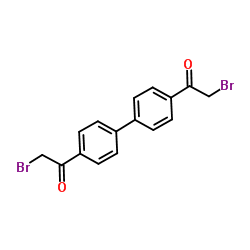
Hemicholinium 3

Hemicholinium 3 structure
|
Common Name | Hemicholinium 3 | ||
|---|---|---|---|---|
| CAS Number | 312-45-8 | Molecular Weight | 574.35 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C24H34Br2N2O4 | Melting Point | 180 °C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Hemicholinium 3Hemicholinium 3 is a competitive inhibitor of the high affinity choline transporter (HACU) with a Ki value of 25 nM. Hemicholinium 3, a neuromuscular blocking agent which inhibits the synthesis and the release of acetylcholine (ACh)[1]. Hemicholinium 3 inhibits the Epibatidine-evoked contraction and [3H]acetylcholine release with IC50s of 897 nM and 693 nM, respectively[2]. |
| Name | 2-[4-[4-(2-hydroxy-4,4-dimethylmorpholin-4-ium-2-yl)phenyl]phenyl]-4,4-dimethylmorpholin-4-ium-2-ol,dibromide |
|---|---|
| Synonym | More Synonyms |
| Description | Hemicholinium 3 is a competitive inhibitor of the high affinity choline transporter (HACU) with a Ki value of 25 nM. Hemicholinium 3, a neuromuscular blocking agent which inhibits the synthesis and the release of acetylcholine (ACh)[1]. Hemicholinium 3 inhibits the Epibatidine-evoked contraction and [3H]acetylcholine release with IC50s of 897 nM and 693 nM, respectively[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Hemicholinium 3 (HC-3) inhibits the presynaptic nicotinic acetylcholine receptors of myenteric neurons. HC-3 inhibits nAChRs located on the terminal region of myenteric neurons of guinea-pig longitudinal muscle strip preparation[2]. Hemicholinium 3 (HC-3) inhibits[3H]choline uptake with the Ki value of 13.3 μM in NCI-H69 cells[3]. HC-3 (1 mM) significantly inhibits cell viability and increases caspase-3/7 activity in NCI-H69 cells[3]. Hemicholinium 3 (Hemicholinium-3) inhibits sodium dependent high affinity choline uptake (IC50=18 nM)[4]. In vivo: Cell Viability Assay[3] Cell Line: The human small cell lung carcinoma cell line NCI-H69 Concentration: 1 mM Incubation Time: Added every day for 2 days. Result: Markedly inhibited cell viability. |
| In Vivo | Hemicholinium-3 impairs spatial learning. HC-3 (2.5, 5.0 μg/rat/ICV; 1 h before training) dose dependently impairs spatial learning[5]. |
| References |
| Melting Point | 180 °C |
|---|---|
| Molecular Formula | C24H34Br2N2O4 |
| Molecular Weight | 574.35 |
| Exact Mass | 572.08900 |
| PSA | 58.92000 |
| InChIKey | OPYKHUMNFAMIBL-UHFFFAOYSA-L |
| SMILES | C[N+]1(C)CCOC(O)(c2ccc(-c3ccc(C4(O)C[N+](C)(C)CCO4)cc3)cc2)C1.[Br-].[Br-] |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 + H311 + H331-H315-H319-H335 |
| Precautionary Statements | P261-P280-P301 + P310-P305 + P351 + P338-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | 23/24/25-36/37/38 |
| Safety Phrases | S26;S45;S36/S37/S39 |
| RIDADR | UN 2811 6.1/PG 2 |
| RTECS | QF2450000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Identification and characterization of ML352: a novel, noncompetitive inhibitor of the presynaptic choline transporter.
ACS Chem. Neurosci. 6(3) , 417-27, (2015) The high-affinity choline transporter (CHT) is the rate-limiting determinant of acetylcholine (ACh) synthesis, yet the transporter remains a largely undeveloped target for the detection and manipulati... |
|
|
Transient Receptor Potential Channel Opening Releases Endogenous Acetylcholine, which Contributes to Endothelium-Dependent Relaxation Induced by Mild Hypothermia in Spontaneously Hypertensive Rat but Not Wistar-Kyoto Rat Arteries.
J. Pharmacol. Exp. Ther. 354 , 121-30, (2015) Mild hypothermia causes endothelium-dependent relaxations, which are reduced by the muscarinic receptor antagonist atropine. The present study investigated whether endothelial endogenous acetylcholine... |
|
|
Four cation-selective transporters contribute to apical uptake and accumulation of metformin in Caco-2 cell monolayers.
J. Pharmacol. Exp. Ther. 352(3) , 519-28, (2015) Metformin is the frontline therapy for type II diabetes mellitus. The oral bioavailability of metformin is unexpectedly high, between 40 and 60%, given its hydrophilicity and positive charge at all ph... |
| Hemicholinium dibromide |
| HC-3 |
| HEMICHOLINIUM 3 |
| EINECS 206-227-3 |
| Hemicholinium 3 dibromide |
| Hemicholinium bromide |
| Hemicholinium-3 bromide |
| Hemicholinium-3 |
| MFCD00011978 |
| hemicholinium |
| Hemicholine |