Glurate
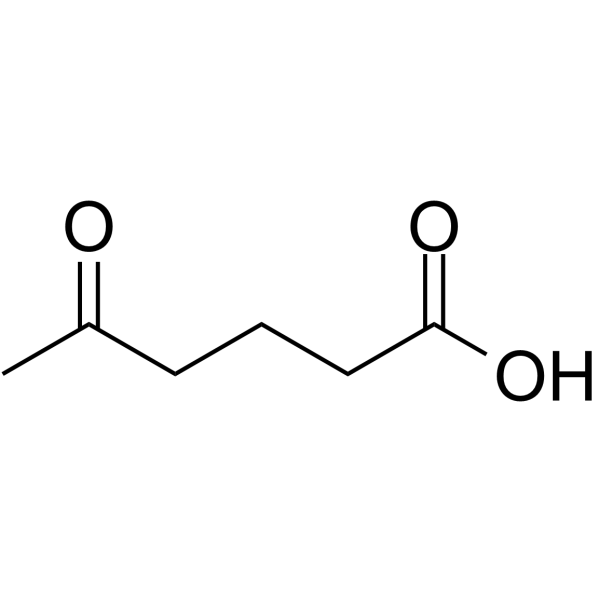
Glurate structure
|
Common Name | Glurate | ||
|---|---|---|---|---|
| CAS Number | 3128-06-1 | Molecular Weight | 130.14200 | |
| Density | 1.09 g/mL at 25 °C(lit.) | Boiling Point | 274-275 °C(lit.) | |
| Molecular Formula | C6H10O3 | Melting Point | 13-14 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | >230 °F | |
Use of GlurateGlurate (4-Acetylbutyric acid; 5-Oxohexanoic acid) can be used to construct antiviral agents (acyclic nucleoside esters) (extracted from patent WO1997030052A1)[1]. |
| Name | 5-oxohexanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Glurate (4-Acetylbutyric acid; 5-Oxohexanoic acid) can be used to construct antiviral agents (acyclic nucleoside esters) (extracted from patent WO1997030052A1)[1]. |
|---|---|
| Related Catalog | |
| References |
[1]. Per Engelhardt, et al. Synthesis of acyclic nucleosides. Patent WO1997030052A1. |
| Density | 1.09 g/mL at 25 °C(lit.) |
|---|---|
| Boiling Point | 274-275 °C(lit.) |
| Melting Point | 13-14 °C(lit.) |
| Molecular Formula | C6H10O3 |
| Molecular Weight | 130.14200 |
| Flash Point | >230 °F |
| Exact Mass | 130.06300 |
| PSA | 54.37000 |
| LogP | 0.83030 |
| Index of Refraction | n20/D 1.4451(lit.) |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2918300090 |
|---|---|
| Summary | 2918300090 other carboxylic acids with aldehyde or ketone function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Identification of 5-hydroxyhexanoic acid, 4-hydroxyheptanoic acid and 4-hydroxyoctanoic acid as new constituents of bacterial polyhydroxyalkanoic acids. Valentin HE, et al.
Appl. Microbiol. Biotechnol. 46(3) , 261-267, (1996)
|
|
|
Proline-like β-turn mimics accessed via Ugi reaction involving monoprotected hydrazines. Krasavin M, et al.
Tetrahedron Lett. 51(10) , 1367-70, (2010)
|
|
|
A versatile and concise route to functionally substituted ?-butyrolactones and spiro-XXX-butyrolactones (lactone annelation) Mandal AK and Jawalkar DG
Tetrahedron Lett. 27.1 , 99-100, (1986)
|
| EINECS 221-511-7 |
| 5-Ketocaproic acid |
| 5-Oxohexanoic Acid |
| 4-Acetylbutyric Acid |
| 4-Acetylbutyricacid |
| 5-Ketohexanoic acid |
| Hexanoic acid,5-oxo |
| 5-oxocaproic acid |
| 4-acetyl-butanoic acid |
| MFCD00004412 |
| 5-oxo-hexanoic acid |
 CAS#:3400-78-0
CAS#:3400-78-0 CAS#:504-02-9
CAS#:504-02-9 CAS#:1577-22-6
CAS#:1577-22-6 CAS#:50-99-7
CAS#:50-99-7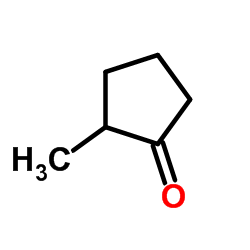 CAS#:1120-72-5
CAS#:1120-72-5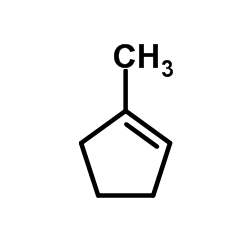 CAS#:693-89-0
CAS#:693-89-0 CAS#:64-19-7
CAS#:64-19-7 CAS#:591-49-1
CAS#:591-49-1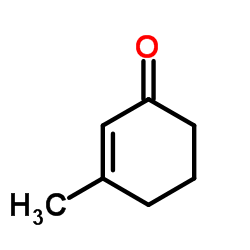 CAS#:1193-18-6
CAS#:1193-18-6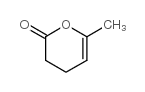 CAS#:3740-59-8
CAS#:3740-59-8 CAS#:70108-70-2
CAS#:70108-70-2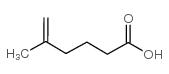 CAS#:55170-74-6
CAS#:55170-74-6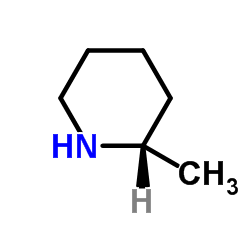 CAS#:3197-42-0
CAS#:3197-42-0 CAS#:539-88-8
CAS#:539-88-8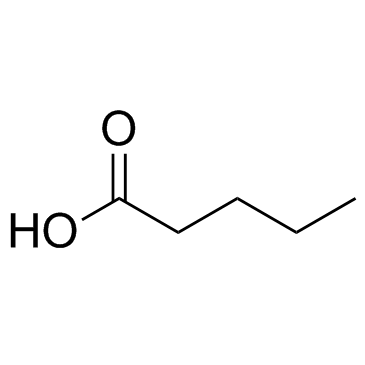 CAS#:109-52-4
CAS#:109-52-4 CAS#:29393-32-6
CAS#:29393-32-6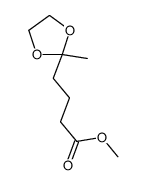 CAS#:29310-39-2
CAS#:29310-39-2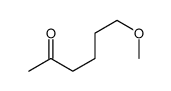 CAS#:29006-00-6
CAS#:29006-00-6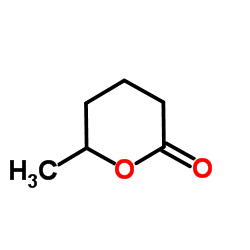 CAS#:823-22-3
CAS#:823-22-3
