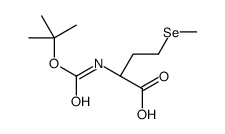
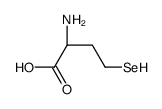
L-Selenomethionine
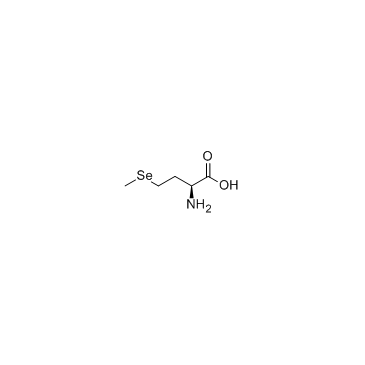
L-Selenomethionine structure
|
Common Name | L-Selenomethionine | ||
|---|---|---|---|---|
| CAS Number | 3211-76-5 | Molecular Weight | 196.106 | |
| Density | N/A | Boiling Point | 320.8±37.0 °C at 760 mmHg | |
| Molecular Formula | C5H11NO2Se | Melting Point | 265 °C | |
| MSDS | Chinese USA | Flash Point | 147.8±26.5 °C | |
| Symbol |



GHS06, GHS08, GHS09 |
Signal Word | Danger | |
Use of L-SelenomethionineL-SelenoMethionine is a major natural food-form of selenium.Target:The median lethal dose (LD50) of L-SelenoMethionine in rats given an intraperitoneal injection was determined to 4.25 mg Se/kg body and thus is comparable to that of selenite or selenate. In mice, the LD50 of L-SelenoMethionine was 8.8 ± 1.37 mg Se/kg, and the minimal lethal dose, 4.0 mg Se/kg, after intravenous injection. |
| Name | L-selenomethionine |
|---|---|
| Synonym | More Synonyms |
| Description | L-SelenoMethionine is a major natural food-form of selenium.Target:The median lethal dose (LD50) of L-SelenoMethionine in rats given an intraperitoneal injection was determined to 4.25 mg Se/kg body and thus is comparable to that of selenite or selenate. In mice, the LD50 of L-SelenoMethionine was 8.8 ± 1.37 mg Se/kg, and the minimal lethal dose, 4.0 mg Se/kg, after intravenous injection. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Boiling Point | 320.8±37.0 °C at 760 mmHg |
|---|---|
| Melting Point | 265 °C |
| Molecular Formula | C5H11NO2Se |
| Molecular Weight | 196.106 |
| Flash Point | 147.8±26.5 °C |
| Exact Mass | 196.995499 |
| PSA | 63.32000 |
| LogP | -0.65 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 18 ° (C=0.5, 2mol/L HCl) |
| InChIKey | RJFAYQIBOAGBLC-BYPYZUCNSA-N |
| SMILES | C[Se]CCC(N)C(=O)O |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS06, GHS08, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 + H331-H373-H410 |
| Precautionary Statements | Missing Phrase - N15.00950417-P260-P304 + P340 + P312-P403 + P233 |
| Hazard Codes | T:Toxic;N:Dangerousfortheenvironment; |
| Risk Phrases | R23/25;R33;R50/53 |
| Safety Phrases | S20/21-S28-S45-S60-S61-S28A |
| RIDADR | UN 3283 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | EK7713840 |
| Packaging Group | I; II; III |
| Hazard Class | 6.1 |
| HS Code | 2922499990 |
|
~98% 
L-Selenomethionine CAS#:3211-76-5 |
| Literature: Barton, Derek H. R.; Bridon, Dominique; Herve, Yolande; Potier, Pierre; Thierry, Josiane; Zard, Samir Z. Tetrahedron, 1986 , vol. 42, # 18 p. 4983 - 4990 |
|
~% 
L-Selenomethionine CAS#:3211-76-5 |
| Literature: Tetrahedron Asymmetry, , vol. 8, # 19 p. 3197 - 3200 |
|
~% 
L-Selenomethionine CAS#:3211-76-5 |
| Literature: Tetrahedron, , vol. 42, # 18 p. 4983 - 4990 |
|
~% 
L-Selenomethionine CAS#:3211-76-5 |
| Literature: Tetrahedron Asymmetry, , vol. 8, # 19 p. 3197 - 3200 |
|
~% 
L-Selenomethionine CAS#:3211-76-5 |
| Literature: Collection of Czechoslovak Chemical Communications, , vol. 33, p. 3910 - 3912 |
|
~% 
L-Selenomethionine CAS#:3211-76-5 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 10, # 21 p. 2471 - 2475 |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression.
Nature 457(7231) , 910-4, (2009) Multiple, complex molecular events characterize cancer development and progression. Deciphering the molecular networks that distinguish organ-confined disease from metastatic disease may lead to the i... |
|
|
Structure of the Ergothioneine-Biosynthesis Amidohydrolase EgtC.
ChemBioChem. 16 , 1490-6, (2015) The ubiquitous sulfur metabolite ergothioneine is biosynthesized by oxidative attachment of a sulfur atom to the imidazole ring of Nα-trimethylhistidine. Most actinobacteria, including Mycobacterium t... |
| L-Selenomethioninum |
| (2S)-2-Amino-4-(methylseleno)butanoic acid |
| (S)-2-Amino-4-(Methylseleno)Butyric Acid |
| MFCD00037210 |
| (2S)-2-Amino-4-(methylselanyl)butanoic acid |
| Selenium-L-methionine |
| Se-methionine |
| SeMet |
| L-selenomethionine zwitterion |
| (S)-2-Amino-4-(methylselanyl)butanoic acid |
| Seleno-L-methionine |
| (2S)-2-amino-4-methylselanylbutanoic acid |
| Butanoic acid, 2-amino-4-(methylseleno)-, (2S)- |
| L(+)-Selenomethionine |
| (S)-2-Amino-4-(methylseleno)butanoic acid |
| L-SelenoMethionine |
| L-(+)-Selenomethionine |
| Selenomethionine |