11-Ketodihydrotestosterone
Modify Date: 2024-01-02 19:28:00
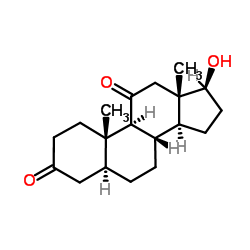
11-Ketodihydrotestosterone structure
|
Common Name | 11-Ketodihydrotestosterone | ||
|---|---|---|---|---|
| CAS Number | 32694-37-4 | Molecular Weight | 304.424 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 453.4±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H28O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 242.1±25.2 °C | |
Use of 11-Ketodihydrotestosterone11-Ketodihydrotestosterone (11-KDHT; 5α-Dihydro-11-keto testosterone) is an endogenous steroid and a metabolite of 11β-Hydroxyandrostenedione. 11-Ketodihydrotestosterone is an active androgen and is also a potent androgen receptor (AR) agonist with a Ki of 20.4 nM and an EC50 of 1.35 nM for human AR. 11-Ketodihydrotestosterone drives gene regulation, protein expression and cell growth in androgen-dependent prostate cancer cells[1][2]. |
| Name | (5α,17β)-17-Hydroxyandrostane-3,11-dione |
|---|---|
| Synonym | More Synonyms |
| Description | 11-Ketodihydrotestosterone (11-KDHT; 5α-Dihydro-11-keto testosterone) is an endogenous steroid and a metabolite of 11β-Hydroxyandrostenedione. 11-Ketodihydrotestosterone is an active androgen and is also a potent androgen receptor (AR) agonist with a Ki of 20.4 nM and an EC50 of 1.35 nM for human AR. 11-Ketodihydrotestosterone drives gene regulation, protein expression and cell growth in androgen-dependent prostate cancer cells[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 20.4 nM (Human androgen receptor)[1] EC50: 1.35 nM (Human androgen receptor)[1] |
| In Vitro | 11-Ketodihydrotestosterone (11-KDHT; 1-10 nM; 24 hours; LNCaP and VCaP cells) treatment induces significant cell proliferation[1]. 11-Ketodihydrotestosterone (11-KDHT; 0.1-10 nM; 7-10 days; LNCaP and VCaP cells) treatment results in the significant upregulation of KLK3, TMPRSS2 and FKBP5 in both LNCaP and VCaP cells, with the exception of KLK3 at 1 nM in LNCaP cells[1]. In PNT2 cells, only 20% of 11β-hydroxyandrostenedione (11OHA4) and 11β-hydroxytestosterone (11OHT) are metabolised with the former yielding 11keto-androstenedione (11KA4), 11-Ketodihydrotestosterone (11-KDHT) and 11β-hydroxy-5α-androstanedione (11OH-5αDIONE) and the latter yielding 11OHA4, 11KT and 11-Ketodihydrotestosterone with downstream products <0.03 μM[2]. In prostate cancer tissue, C11-oxy C19 metabolites at significantly higher levels than the C19 steroids are detected, with unconjugated 11-Ketodihydrotestosterone, 11KT and 11OHA4 levels ranging between 13 and 37.5 ng/g. Analyses of total steroid levels in plasma show significant levels of 11OHA4 (≈230-440 nM), 11KT (≈250-390 nM) and 11-Ketodihydrotestosterone (≈19 nM)[2]. Cell Proliferation Assay[1] Cell Line: LNCaP and VCaP cells Concentration: 0.1 nM, 1 nM or 10 nM Incubation Time: 7 days (LNCaP cells) or 10 days (VCaP cells) Result: Induced significant cell proliferation. RT-PCR[1] Cell Line: LNCaP and VCaP cells Concentration: 1 nM, 10 nM Incubation Time: 24 hours Result: Resulted in the significant upregulation of KLK3, TMPRSS2 and FKBP5 in both LNCaP (Fig 3) and VCaP (Fig 4) cells. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 453.4±45.0 °C at 760 mmHg |
| Molecular Formula | C19H28O3 |
| Molecular Weight | 304.424 |
| Flash Point | 242.1±25.2 °C |
| Exact Mass | 304.203857 |
| LogP | 1.84 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.546 |
| Storage condition | -20°C |
| Androstane-3,11-dione, 17-hydroxy-, (5α,17β)- |
| (5α,17β)-17-Hydroxyandrostane-3,11-dione |
| 5α-Androstane-3,11-dione, 17β-hydroxy- |