5-methyl-2-thiouridine
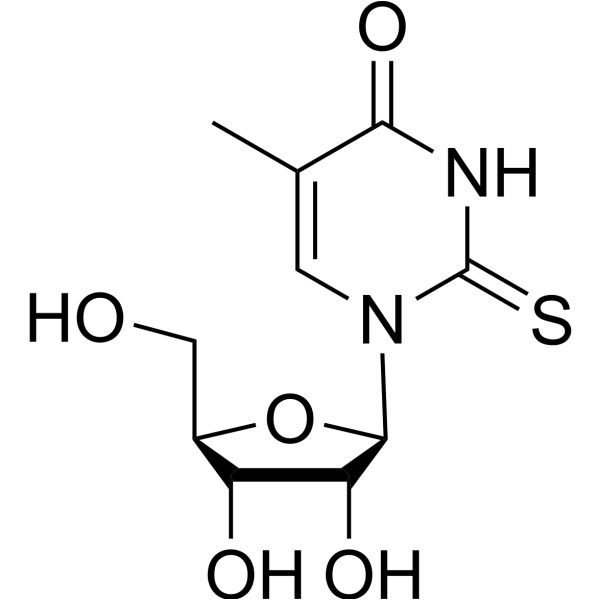
5-methyl-2-thiouridine structure
|
Common Name | 5-methyl-2-thiouridine | ||
|---|---|---|---|---|
| CAS Number | 32738-09-3 | Molecular Weight | 274.29 | |
| Density | 1.63g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C10H14N2O5S | Melting Point | 216-219 °C | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of 5-methyl-2-thiouridine5-Methyl-2-thiouridine (2-Thio-5-methyluridine) is a purine nucleoside analogue. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc[1]. |
| Name | 5-methyl-2-thiouridine |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Methyl-2-thiouridine (2-Thio-5-methyluridine) is a purine nucleoside analogue. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.63g/cm3 |
|---|---|
| Melting Point | 216-219 °C |
| Molecular Formula | C10H14N2O5S |
| Molecular Weight | 274.29 |
| Exact Mass | 274.06200 |
| PSA | 140.06000 |
| Index of Refraction | 1.703 |
| InChIKey | SNNBPMAXGYBMHM-JXOAFFINSA-N |
| SMILES | Cc1cn(C2OC(CO)C(O)C2O)c(=S)[nH]c1=O |
| RIDADR | NONH for all modes of transport |
|---|---|
| WGK Germany | 3 |
| HS Code | 2934999090 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon.
Proc. Natl. Acad. Sci. U. S. A. 82 , 4905-4909, (1985) Proton NMR analyses have been made to elucidate the conformational characteristics of modified nucleotides as found in the first position of the anticodon of tRNA [derivatives of 5-methyl-2-thiouridin... |
|
|
CD spectra of 5-methyl-2-thiouridine in tRNA-Met-f from an extreme thermophile.
Nucleic Acids Res. 3 , 1703-1713, (1976) 5-Methyl-2-thiouridine (S) in tRNA-Met-f from an extreme thermophile is located in the TpsiC region, replacing T, and has a positive CD band centered at 310 nm. Upon heating, the profiles of the chang... |
|
|
Stabilizing contributions of sulfur-modified nucleotides: crystal structure of a DNA duplex with 2'-O-[2-(methoxy)ethyl]-2-thiothymidines.
Nucleic Acids Res. 33 , 5297-5307, (2005) Substitution of oxygen atoms by sulfur at various locations in the nucleic acid framework has led to analogs such as the DNA phosphorothioates and 4'-thio RNA. The phosphorothioates are excellent mimi... |
| 2-Sulfamyl-4-methyl-phenyl-thioharnstoff |
| 5-Methyl-2-thio-uridin |
| 5-methyl-2-thioureido-benzenesulfonamide |
| 5-Methyl-1-pentofuranosyl-2-thioxo-2,3-dihydro-4(1H)-pyrimidinone |
| 4-Methyl-2-sulphamylphenylthiourea |
| Benzenesulfonamide,2-[(aminothioxomethyl)amino]-5-methyl |
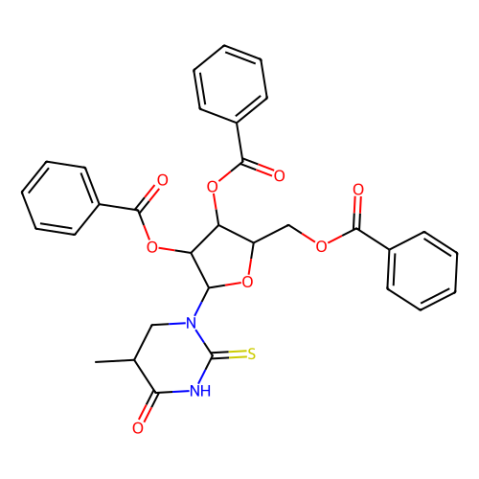 CAS#:75921-08-3
CAS#:75921-08-3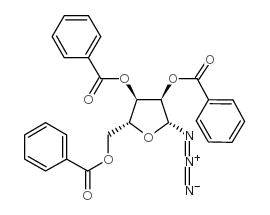 CAS#:7408-41-5
CAS#:7408-41-5