3-Methylvaline
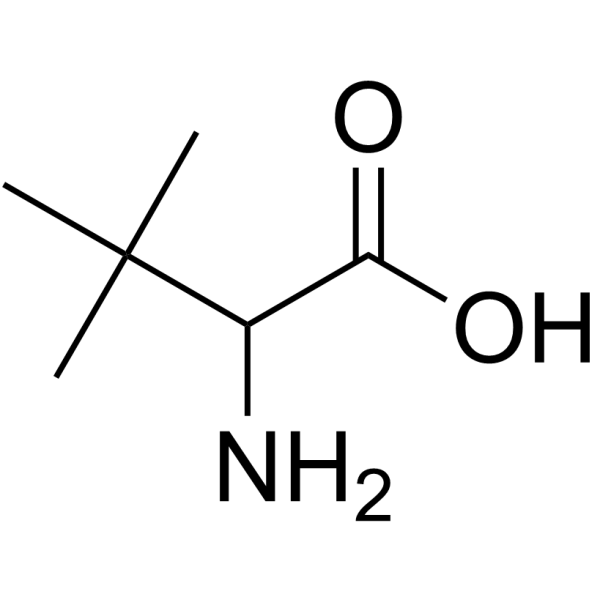
3-Methylvaline structure
|
Common Name | 3-Methylvaline | ||
|---|---|---|---|---|
| CAS Number | 33105-81-6 | Molecular Weight | 131.173 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 217.7±23.0 °C at 760 mmHg | |
| Molecular Formula | C6H13NO2 | Melting Point | 299-301ºC | |
| MSDS | USA | Flash Point | 85.5±22.6 °C | |
Use of 3-Methylvaline2-Amino-3,3-dimethylbutanoic acid is a leucine derivative[1]. |
| Name | 2-amino-3,3-dimethylbutanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Amino-3,3-dimethylbutanoic acid is a leucine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 217.7±23.0 °C at 760 mmHg |
| Melting Point | 299-301ºC |
| Molecular Formula | C6H13NO2 |
| Molecular Weight | 131.173 |
| Flash Point | 85.5±22.6 °C |
| Exact Mass | 131.094635 |
| PSA | 63.32000 |
| LogP | 0.55 |
| Vapour Pressure | 0.1±0.9 mmHg at 25°C |
| Index of Refraction | 1.464 |
| Storage condition | Store at RT. |
| Water Solubility | H2O: 0.1 g/mL, clear, colorless | soluble |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
|
Highly selective asymmetric acetate aldol reactions of an N-acetyl thiazolidinethione reagent.
Org. Lett. 6 , 23-25, (2004) [reaction: see text] A highly diastereoselective acetate aldol reaction that uses a tert-leucine-derived thiazolidinethione auxiliary and dichlorophenylborane has been developed. The reaction proceeds... |
|
|
Scaleable catalytic asymmetric Strecker syntheses of unnatural alpha-amino acids.
Nature 461(7266) , 968-70, (2009) Alpha-amino acids are the building blocks of proteins and are widely used as components of medicinally active molecules and chiral catalysts. Efficient chemo-enzymatic methods for the synthesis of ena... |
|
|
An efficient and selective enzymatic oxidation system for the synthesis of enantiomerically pure D-tert-leucine.
Org. Lett. 5(20) , 3649-50, (2003) [reaction: see text] d-tert-Leucine was prepared with an enantiomeric excess of >99% by an enzyme-catalyzed oxidative resolution of the racemic mixture of dl-tert-leucine with use of leucine dehydroge... |
| L-2-(tert-butyl)glycine |
| tert-leucine |
| L-Tert-Leucine |
| L-tert-leucin |
| (S)-2-Amino-3,3-dimethylbutyric acid |
| LEUCINE,L |
| 2-tert-butyl-glycine |
| DL-Tert-Leucine |
| 3-Methylvaline |
| (S)-2-Amino-4-methylpentanoic acid |
| T-BUTYL GLYCINE |
| DL-tert-Butylglycine |
| L-2-amino-3,3-dimethylbutanoic acid |
| (2S)-2-amino-3,3-dimethylbutanoic acid |
| 3-Methyl-L-valine |
| L-Valine, 3-methyl- |
| L-2-tert-Butylglycine |
| Valine, 3-methyl- |
| (S)-2-Amino-3,3-dimethylbutanoic acid |
| 3-Methyl valine |
| t-Butylglycine |
| h-dl-tle-oh |
| L-α-tert-Butylglycine |
| dl-t-butylglycine |
| L-tert |
| H-L-LEU-OH |
| QVYZX1&1&1 &&L or S Form |
| 2-Amino-3,3-dimethylbutanoic acid |
| 2-amino-3,3-dimethyl-butyric acid |
| tert-butylglycine |
| MFCD00065933 |
| H-Tle-OH |
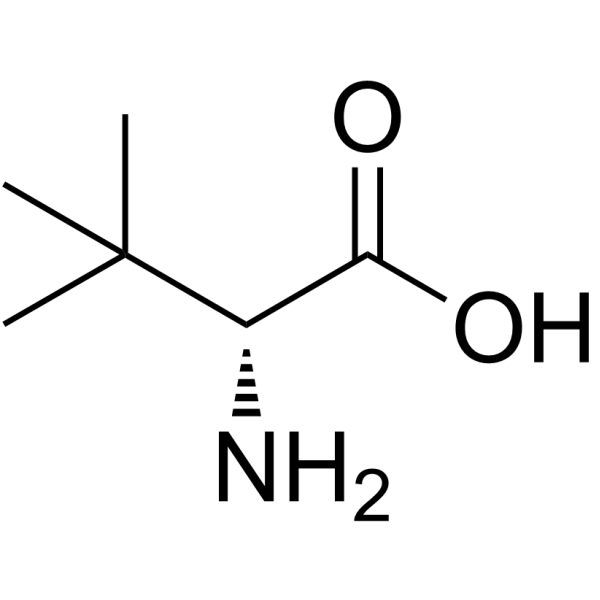 CAS#:26782-71-8
CAS#:26782-71-8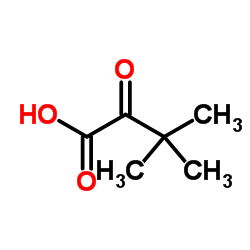 CAS#:815-17-8
CAS#:815-17-8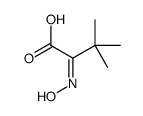 CAS#:62965-34-8
CAS#:62965-34-8 CAS#:38559-30-7
CAS#:38559-30-7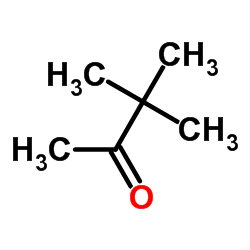 CAS#:75-97-8
CAS#:75-97-8 CAS#:630-19-3
CAS#:630-19-3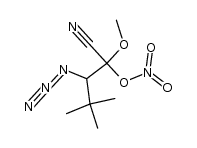 CAS#:1025821-45-7
CAS#:1025821-45-7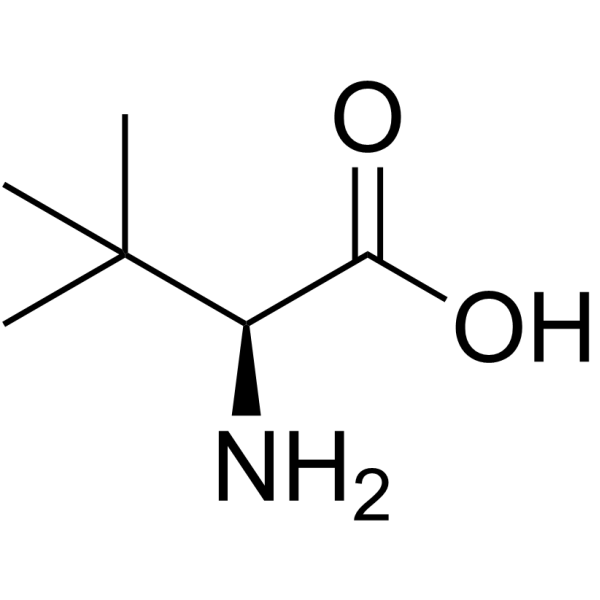 CAS#:20859-02-3
CAS#:20859-02-3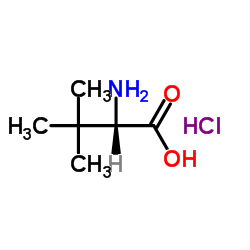 CAS#:139163-43-2
CAS#:139163-43-2 CAS#:169961-91-5
CAS#:169961-91-5 CAS#:71953-55-4
CAS#:71953-55-4 CAS#:68222-59-3
CAS#:68222-59-3 CAS#:92571-59-0
CAS#:92571-59-0 CAS#:92571-61-4
CAS#:92571-61-4 CAS#:3850-31-5
CAS#:3850-31-5
