Dithianon
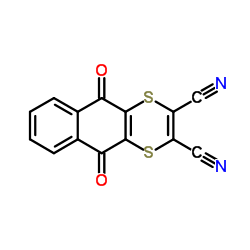
Dithianon structure
|
Common Name | Dithianon | ||
|---|---|---|---|---|
| CAS Number | 3347-22-6 | Molecular Weight | 296.324 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 402.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C14H4N2O2S2 | Melting Point | 220ºC | |
| MSDS | Chinese USA | Flash Point | 197.0±28.7 °C | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of DithianonDithianon is a broad-spectrum anthraquinone fungicide with good adherence to the surface of leaves and fruits. Dithianon is used to control several several fungal of some fruits and vegetables, as anthracnose (Colletotrichum sp., Elsinoe ampelina), mildew (Plasmopara viticola), phomopsis (Phomopsis viticola), among others[1][2]. |
| Name | dithianon |
|---|---|
| Synonym | More Synonyms |
| Description | Dithianon is a broad-spectrum anthraquinone fungicide with good adherence to the surface of leaves and fruits. Dithianon is used to control several several fungal of some fruits and vegetables, as anthracnose (Colletotrichum sp., Elsinoe ampelina), mildew (Plasmopara viticola), phomopsis (Phomopsis viticola), among others[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Reactive oxygen species (ROS)[1] Colletotrichum sp.; Elsinoe ampelina; Plasmopara viticola; Phomopsis viticola[1] |
| In Vitro | When exponentially aerobic growing cells of S. cerevisiae are submitted to acute Dithianon treatment they loss cell wall and membrane integrity, dying by necrosis, and this behavior is associated to a depletion of reduced proteic and non-proteic thiol groups. An important increase of cellular reactive oxygen species (ROS) associated to mitochondrial membrane potential modifications on Dithianon treated cells are also detected[1]. In filamentous fungus, Dithianon inhibits mycelial growth and conidial germination. Studies on Ehrlich ascites carcinoma and yeast cells showed that Dithianon inhibits respiration and fermentation affecting several thiol enzymes of the glycolytic pathway as hexokinase, phosphofructokinase, and glyceraldeyde-3-phosphate dehydrogenase[1]. Dithianon has in vitro cytotoxic effect and affect cell transforming activity of BLAB/c 3 T3 cells[1]. |
| In Vivo | The activity of testosterone hydroxylase of liver microsomes derived from male mice is increased when they are treated with acute doses of Dithianon, while in females an inactivating effect is observed[1]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 402.1±45.0 °C at 760 mmHg |
| Melting Point | 220ºC |
| Molecular Formula | C14H4N2O2S2 |
| Molecular Weight | 296.324 |
| Flash Point | 197.0±28.7 °C |
| Exact Mass | 295.971405 |
| PSA | 138.20000 |
| LogP | 1.86 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.776 |
| Storage condition | 0-6°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H410 |
| Precautionary Statements | P273-P501 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful;N:Dangerousfortheenvironment; |
| Risk Phrases | R22;R50/53 |
| Safety Phrases | S24-S60-S61 |
| RIDADR | UN 3077 |
| RTECS | QL0700000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
Effect of thiol blocking agents on the binding of T3 and T4 in rat liver nuclear extract.
Acta Endocrinol. 98(1) , 68-72, (1981) Liver nuclei bind thyroid hormones (T3 and T4) with different binding affinities. In out studies it was estimated that the apparent association constant (Ka) in the liver nuclei for T3 was 8.8 X 10(10... |
|
|
Identification of ABC transporter genes of Fusarium graminearum with roles in azole tolerance and/or virulence.
PLoS ONE 8 , e79042, (2013) Fusarium graminearum is a plant pathogen infecting several important cereals, resulting in substantial yield losses and mycotoxin contamination of the grain. Triazole fungicides are used to control di... |
|
|
In vitro cytotoxic and cell transforming activities exerted by the pesticides cyanazine, dithianon, diflubenzuron, procymidone, and vinclozolin on BALB/c 3T3 cells.
Environ. Mol. Mutagen. 21(1) , 81-6, (1993) Cytotoxic and cell transforming activities of the pesticides cyanazine, diflubenzuron, dithianon, procymidone, and vinclozolin were investigated in vitro by utilizing the BALB/c 3T3 cell transformatio... |
| 2,3-Dicyano-1,4-dithiaanthraquinone |
| Thynon |
| 5,10-Dioxo-5,10-dihydronaphtho[2,3-b][1,4]dithiine-2,3-dicarbonitrile |
| Caswell No. 349B |
| Delan-col |
| 5,10-dioxobenzo[g][1,4]benzodithiine-2,3-dicarbonitrile |
| Dithianon |
| Delan |
| 5,10-dihydro-5,10-dioxonaphtho[2,3-b]-1,4-dithiine-2,3-dicarbonitrile |
| EINECS 222-098-6 |
| DITHIANONE |
| MFCD00055492 |
| Delan WP |
| Naphtho[2,3-b]-1,4-dithiin-2,3-dicarbonitrile, 5,10-dihydro-5,10-dioxo- |
| Stauffer MV 119A |
| Stauffer MV-119A |
| Merkdelan |
| 2,3-dicyano-1,4-dithia-anthraquinone |
| 5,10-Dihydro-5,10-dioxonaphtho[2,3-b]-1,4-dithiin-2,3-dicarbonitrile |

