8,11,14-Icosatriynoic acid
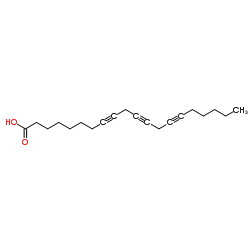
8,11,14-Icosatriynoic acid structure
|
Common Name | 8,11,14-Icosatriynoic acid | ||
|---|---|---|---|---|
| CAS Number | 34262-64-1 | Molecular Weight | 300.435 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 469.6±40.0 °C at 760 mmHg | |
| Molecular Formula | C20H28O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 220.4±22.0 °C | |
Use of 8,11,14-Icosatriynoic acid8,11,14-Eicosatriynoic Acid, as an inhibitor of prostaglandins, leukotriene biosynthesis and arachidonic acid-induced platelet aggregation, blocks human 12-lipoxygenase (12-LO), cyclooxygenation The enzyme (COX) and 5-lipoxygenase (5-LO) IC50 values were 0.46 μM, 14 μM and 25 μM, respectively. In addition, it inhibits the action of slow-reacting substances of allergic reactions with IC50 values of 10 μM [1,2]. Lipoxygenase is widely found in fungi, plants and animals, and its content is very high. 12-LO is involved in many important disease states and may play a role in oxidative glutamate toxicity. COX enzymes play complex roles in human physiology and pathology involving the neuronal, immune, renal, cardiovascular, gastrointestinal and reproductive systems. COX enzymes are blocked by aspirin and various other NSAIDs, making them clinically important [3]. 5-LO is involved in cancer pathology. It is expressed by a variety of cancer cells, including colon, lung, breast, and prostate cancers, and promotes cancer cell growth and neovascularization. In vitro: As of now, in vitro studies of 8,11,14-eicosatriynoic acid are still under development. In vivo: To date, in vivo studies of 8,11,14-eicosatriynoic acid are in the development stage. References: [1]. Goetz, J., Sprecher, H., Cornwell, D. and Panganamala, R . Inhibition of prostaglandin biosynthesis by triynoic acid. . prostaglandins. 1976;12(2):187-192. [2]. Sun, F., McGuire, J., Morton, D., Pike, J., Sprecher, H. and Kunau, W. Inhibition of platelet arachidonic acid 12-lipoxygenase by acetylenic acid compounds effect. prostaglandins. 1981;21(2):333-343. [3]. Fitzpatrick, F. Cyclooxygenase: regulation and function. Current Drug Design. 2004;10(6):577-588. |
| Name | 8,11,14-Eicosatriynoic Acid |
|---|---|
| Synonym | More Synonyms |
| Description | 8,11,14-Eicosatriynoic Acid, as an inhibitor of prostaglandins, leukotriene biosynthesis and arachidonic acid-induced platelet aggregation, blocks human 12-lipoxygenase (12-LO), cyclooxygenation The enzyme (COX) and 5-lipoxygenase (5-LO) IC50 values were 0.46 μM, 14 μM and 25 μM, respectively. In addition, it inhibits the action of slow-reacting substances of allergic reactions with IC50 values of 10 μM [1,2]. Lipoxygenase is widely found in fungi, plants and animals, and its content is very high. 12-LO is involved in many important disease states and may play a role in oxidative glutamate toxicity. COX enzymes play complex roles in human physiology and pathology involving the neuronal, immune, renal, cardiovascular, gastrointestinal and reproductive systems. COX enzymes are blocked by aspirin and various other NSAIDs, making them clinically important [3]. 5-LO is involved in cancer pathology. It is expressed by a variety of cancer cells, including colon, lung, breast, and prostate cancers, and promotes cancer cell growth and neovascularization. In vitro: As of now, in vitro studies of 8,11,14-eicosatriynoic acid are still under development. In vivo: To date, in vivo studies of 8,11,14-eicosatriynoic acid are in the development stage. References: [1]. Goetz, J., Sprecher, H., Cornwell, D. and Panganamala, R . Inhibition of prostaglandin biosynthesis by triynoic acid. . prostaglandins. 1976;12(2):187-192. [2]. Sun, F., McGuire, J., Morton, D., Pike, J., Sprecher, H. and Kunau, W. Inhibition of platelet arachidonic acid 12-lipoxygenase by acetylenic acid compounds effect. prostaglandins. 1981;21(2):333-343. [3]. Fitzpatrick, F. Cyclooxygenase: regulation and function. Current Drug Design. 2004;10(6):577-588. |
|---|---|
| Related Catalog |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 469.6±40.0 °C at 760 mmHg |
| Molecular Formula | C20H28O2 |
| Molecular Weight | 300.435 |
| Flash Point | 220.4±22.0 °C |
| Exact Mass | 300.208923 |
| PSA | 37.30000 |
| LogP | 5.85 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.507 |
| InChIKey | QLLIWTBTVOPGCM-UHFFFAOYSA-N |
| SMILES | CCCCCC#CCC#CCC#CCCCCCCC(=O)O |
|
~88% 
8,11,14-Icosatr... CAS#:34262-64-1 |
| Literature: Groza; Ivanov; Myagkova Russian Journal of Bioorganic Chemistry, 1998 , vol. 24, # 6 p. 401 - 404 |
|
~% 
8,11,14-Icosatr... CAS#:34262-64-1 |
| Literature: Groza; Ivanov; Myagkova Russian Journal of Bioorganic Chemistry, 1998 , vol. 24, # 6 p. 401 - 404 |
|
~% 
8,11,14-Icosatr... CAS#:34262-64-1 |
| Literature: Groza; Ivanov; Myagkova Russian Journal of Bioorganic Chemistry, 1998 , vol. 24, # 6 p. 401 - 404 |
|
~% 
8,11,14-Icosatr... CAS#:34262-64-1 |
| Literature: Groza; Ivanov; Myagkova Russian Journal of Bioorganic Chemistry, 1998 , vol. 24, # 6 p. 401 - 404 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| 8,11,14-Eicosatriinsaeure |
| 8,11,14-Icosatriynoic acid |
| 8,11,14-Eicosatriynoic acid |