Ticarcillin
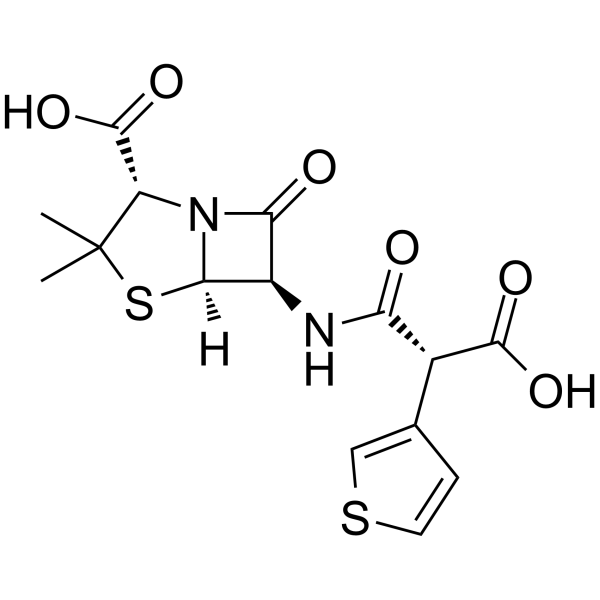
Ticarcillin structure
|
Common Name | Ticarcillin | ||
|---|---|---|---|---|
| CAS Number | 34787-01-4 | Molecular Weight | 384.427 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 768.3±60.0 °C at 760 mmHg | |
| Molecular Formula | C15H16N2O6S2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 418.4±32.9 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of TicarcillinTicarcillin is a semisynthetic, extended-spectrum, carboxypenicillin antibacterial agent, and is active against gram-positive cocci, including streptococci and staphylococci. Ticarcillin is also effective against most gram-negative organisms, including Pseudomonas aeruginosa. Ticarcillin can be used in lower respiratory tract infections, skin and skin structure infections, urinary tract infections, and intraabdominal infections research[1][2][3]. |
| Name | ticarcillin |
|---|---|
| Synonym | More Synonyms |
| Description | Ticarcillin is a semisynthetic, extended-spectrum, carboxypenicillin antibacterial agent, and is active against gram-positive cocci, including streptococci and staphylococci. Ticarcillin is also effective against most gram-negative organisms, including Pseudomonas aeruginosa. Ticarcillin can be used in lower respiratory tract infections, skin and skin structure infections, urinary tract infections, and intraabdominal infections research[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Ticarcillin treatment (10 mg/L; 18 h) shows antibacterial activities[1]. Cell Viability Assay[2] Cell Line: Ps. aeruginosa Concentration: 10 mg/L Incubation Time: 18 hours Result: Showed the MIC for Ticarcillin against strain between 6.3-12.5 mg/L. |
| In Vivo | Ticarcillin (Subcutaneous injection; 50, 100, 200 mg/kg; once) treatment shows a dose-dependent antibacterial effect[2]. Ticarcillin (Subcutaneous injection; 50, 100, 200 mg/kg; once) treatment shows excellent pharmacokinetic assessment[2]. Animal Model: Swiss type male mice injected with Ps. aeruginosa[2] Dosage: 50, 100, 200 mg/kg Administration: Subcutaneous injection; 50, 100, 200 mg/kg; once Result: Increased effect significantly (0.001 < P < 0.0025) with increasing dose. Animal Model: Swiss type male mice injected with Ps. aeruginosa[2] Dosage: 50, 100, 200 mg/kg Administration: Subcutaneous injection; 50, 100, 200 mg/kg; once Result: Dose (mg/kg) AUCa (mg/kg) Kelb (h-1) T1/2 (min) Ticarcillin 50 8.76 (0.72) 1.57 (0.18) 26 100 26.14 (0.60) 1.53 (0.11) 27 200 54.56 (2.90) 1.47 (0.12) 28 |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 768.3±60.0 °C at 760 mmHg |
| Molecular Formula | C15H16N2O6S2 |
| Molecular Weight | 384.427 |
| Flash Point | 418.4±32.9 °C |
| Exact Mass | 384.044983 |
| PSA | 177.55000 |
| LogP | 0.69 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.694 |
| InChIKey | OHKOGUYZJXTSFX-KZFFXBSXSA-N |
| SMILES | CC1(C)SC2C(NC(=O)C(C(=O)O)c3ccsc3)C(=O)N2C1C(=O)O |
| Storage condition | 2-8°C |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H317-H334 |
| Precautionary Statements | P261-P280-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R42/43 |
| Safety Phrases | 22-36/37-45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Methyl gallate from Galla rhois successfully controls clinical isolates of Salmonella infection in both in vitro and in vivo systems.
PLoS ONE 9(7) , e102697, (2014) Galla rhois is a commonly used traditional medicine for the treatment of pathogenic bacteria in Korea as well as in other parts of Asia. Methyl gallate (MG), a major component of Galla Rhois, exhibits... |
|
|
Genetic characterization of oropharyngeal trichomonad isolates from wild birds indicates that genotype is associated with host species, diet and presence of pathognomonic lesions.
Avian Pathol. 43(6) , 535-46, (2014) Oropharyngeal trichomonad isolates of wild birds from Spain were studied. A total of 1688 samples (1214 of predator birds and 474 of prey species) from wildlife recovery centres and scientific bird-ri... |
|
|
Characterization of Acinetobacter baumannii clinical isolates carrying bla(OXA-23) carbapenemase and 16S rRNA methylase armA genes in Yemen.
Microb. Drug Resist. 20(6) , 604-9, (2014) The aim of this study was to investigate the molecular support of resistance to carbapenems, aminoglycosides, and fluoroquinolones in Acinetobacter baumannii clinical isolates collected from Yemen hos... |
| (2S,5R,6R)-6-{[(2R)-2-carboxy-2-(thiophen-3-yl)acetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| Temocillin Disodium |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid,6-[(carboxy-3-thienylacetyl)amino]-3,3-dimethyl-7-oxo-,[2S-[2a,5a,6b(S*)]] |
| 6-[D(-)-a-Carboxy-3-thienylacetamido]penicillanic Acid |
| EINECS 252-213-5 |
| (2S,5R,6R)-6-{[(2R)-2-Carboxy-2-(3-thienyl)acetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| Ticarcillin |
| (2S,5R,6R)-6-[[(2R)-2-carboxy-2-thiophen-3-yl-acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| 6-[D-(-)-a-Carboxy-3-thienylacetamido]penicillanic acid |
| a-Carboxy-3-thienylmethylpenicillin |
| N-(2-Carboxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-6-yl)-3-thiophenemalonamic Acid |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-2-carboxy-2-(3-thienyl)acetyl]amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)- |
| TRIARCILLIN |
| MFCD00864998 |
| Ticcarcillin acid |