Zebularine
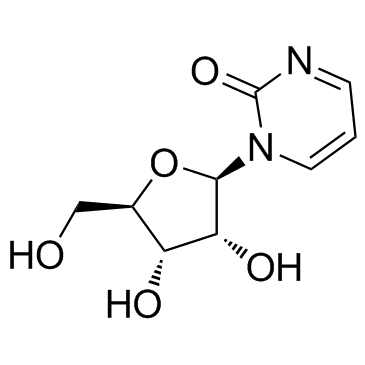
Zebularine structure
|
Common Name | Zebularine | ||
|---|---|---|---|---|
| CAS Number | 3690-10-6 | Molecular Weight | 228.202 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 499.0±55.0 °C at 760 mmHg | |
| Molecular Formula | C9H12N2O5 | Melting Point | 160-162?C | |
| MSDS | Chinese USA | Flash Point | 255.6±31.5 °C | |
Use of ZebularineZebularine (NSC309132; 4-Deoxyuridine) is a DNA methyltransferase inhibitor; also an inhibitor of cytidine deaminase with a Ki of 0.95 μM. |
| Name | zebularine |
|---|---|
| Synonym | More Synonyms |
| Description | Zebularine (NSC309132; 4-Deoxyuridine) is a DNA methyltransferase inhibitor; also an inhibitor of cytidine deaminase with a Ki of 0.95 μM. |
|---|---|
| Related Catalog | |
| Target |
DNMT1 DNMT3a/3L Cytidine deaminase:0.95 μM (Ki) Autophagy |
| In Vitro | Zebularine exerts its demethylation activity by stabilizing the binding of DNMTs to DNA, hindering the methylation and decreasing the dissociation, thereby trapping the enzyme and preventing turnover even at other sites. zebularine enhances tumor cell chemo- and radiosensitivity and has antimitogenic and angiostatic activities[1]. Zebularine inhibits DNA methylation and reactivates a gene previously silenced by methylation. Zebularine induces the myogenic phenotype in 10T1/2 cells, which is a phenomenon unique to DNA methylation inhibitors. Zebularine reactivates a silenced p16 gene and demethylated its promoter region in T24 bladder carcinoma cells[2]. Zebularine treatment inhibits cell growth in a dose and time dependent manner with an IC50 of ∼100 μM and 150 μM in MDA-MB-231 and MCF-7 cells, respectively, on 96 h exposure. At high doses zebularine induced changes in apoptotic proteins in a cell line specific manner manifested by alteration in caspase-3, Bax, Bcl2 and PARP cleavage[3]. Zebularine is also a potent competitive inhibitor of the enzyme CR deaminase. The Ki for zebularine is 0.95μM[4]. |
| In Vivo | Zebularine is only slightly cytotoxic to tumor-bearing mice. Compared with those in control mice, tumor volumes are statistically significantly reduced in mice treated with high-dose zebularine administered by intraperitoneal injection or by oral gavage[2]. Zebularine also enhances the survival time of mice with L1210 leukemia treated with 5-AZA-CdR. About 27% of the mice treated with this drug combination has a survival time longer than the mice treated with only 5-AZA-CdR[4]. |
| Cell Assay | For methylation analysis, 10T1/2 cells and T24 cells are treated with the various concentrations of zebularine. For 10T1/2 cells, the medium is changed 24 hours after the initial drug treatment, whereas for T24 cells, the medium is changed 24 hours or 48 hours after the initial drug treatment. DNA and RNA are harvested from 10T1/2 cells 72 hours after initial drug treatment and from T24 cells 96 hours after initial drug treatment. The methylation status of the indicated DNA regions is measured in two separate and independent experiments, both of which are done in duplicate[2]. |
| Animal Admin | EJ6 cells (5 × 105/injection) suspended in PBS are inoculated subcutaneously into the right and left flanks (along the midaxillary lines) of 4- to 6-week-old male BALB/c nu/nu mice. Mice (n=30) are randomLy divided into six groups (intraperitoneal control, oral control, intraperitoneal zebularine at 500 mg/kg, oral zebularine at 500 mg/kg, intraperitoneal zebularine at 1000 mg/kg, and oral zebularine at 1000 mg/kg). Each group consisted of five mice (at least six tumors per group; one or two mice per group are randomLy killed at earlier time points to establish a time course of expression). After 2–3 weeks and after macroscopic tumors (50–200 mm3) had formed, zebularine or control treatments are initiated. Zebularine, at doses of 500 mg/kg or 1000 mg/kg, is administered daily by intraperitoneal injection or oral gavage in a solution of 0.45% saline over a period of 18 days[2]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 499.0±55.0 °C at 760 mmHg |
| Melting Point | 160-162?C |
| Molecular Formula | C9H12N2O5 |
| Molecular Weight | 228.202 |
| Flash Point | 255.6±31.5 °C |
| Exact Mass | 228.074615 |
| PSA | 104.81000 |
| LogP | -1.31 |
| Appearance of Characters | solid |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.697 |
| InChIKey | RPQZTTQVRYEKCR-WCTZXXKLSA-N |
| SMILES | O=c1ncccn1C1OC(CO)C(O)C1O |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 16 mg/mL, soluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UW7539720 |
|
The potential of clofarabine in MLL-rearranged infant acute lymphoblastic leukaemia.
Eur. J. Cancer 51 , 2008-21, (2015) MLL-rearranged acute lymphoblastic leukaemia (ALL) in infants is the most difficult-to-treat type of childhood ALL, displaying a chemotherapy-resistant phenotype, and unique histone modifications, gen... |
|
|
DNA Methylation Inhibitor Zebularine Confers Stroke Protection in Ischemic Rats.
Transl. Stroke Res. 6 , 296-300, (2015) 5-Aza-deoxycytidine (5-aza-dC) confers neuroprotection in ischemic mice by inhibiting DNA methylation. Zebularine is another DNA methylation inhibitor, less toxic and more stable in aqueous solutions ... |
|
|
Light signaling controls nuclear architecture reorganization during seedling establishment.
Proc. Natl. Acad. Sci. U. S. A. 112 , E2836-44, (2015) The spatial organization of chromatin can be subject to extensive remodeling in plant somatic cells in response to developmental and environmental signals. However, the mechanisms controlling these dy... |
| 4-Deoxyuridine |
| 1--D-Ribofuranosyl-2(1H)-pyrimidinone |
| 4-Deoyuridine |
| 2(1H)-PyriMidinone,1-b-D-ribofuranosyl |
| 1-(β-D-Ribofuranosyl)pyrimidin-2(1H)-one |
| 2(1H)-Pyrimidinone, 1-β-D-ribofuranosyl- |
| Pyrimidin-2-one b-Ribofuranoside |
| Zebularine |
| [14C]-Zebularine |
| 1-b-D-Ribofuranosyl-2(1H)-pyrimidinone |
| 2(1H)-Pyrimidinone, 1-.β.-D-ribofuranosyl- |
| Pyrimidin-2-one-D-Ribofuranoside |
| 1-(β-D-Ribofuranosyl)-2(1H)-pyrimidinone |

![[3,4-diacetyloxy-5-(2-oxopyrimidin-1-yl)oxolan-2-yl]methyl acetate structure](https://image.chemsrc.com/caspic/091/63500-40-3.png)