CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
EJ3500500
-
CHEMICAL NAME :
-
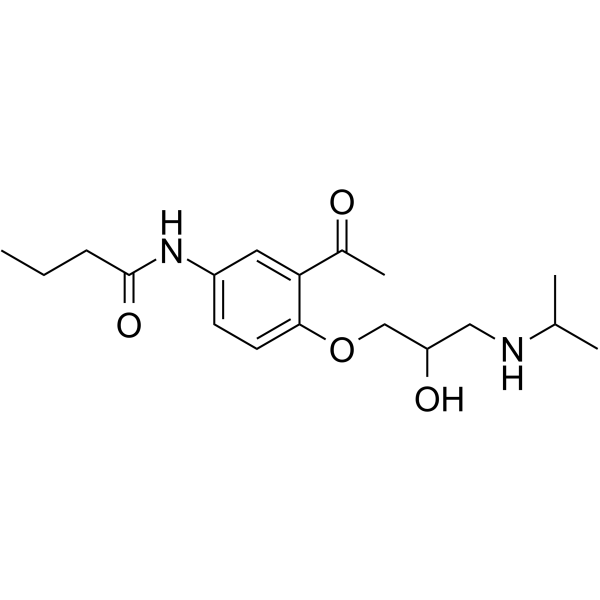
Butanamide, N-(3-acetyl-4-(2-hydroxy-3-((1-methylethyl)amino)prop oxy)phenyl)-, (+-)-
-
CAS REGISTRY NUMBER :
-
37517-30-9
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
8
-
MOLECULAR FORMULA :
-
C18-H28-N2-O4
-
MOLECULAR WEIGHT :
-
336.48
-
WISWESSER LINE NOTATION :
-
3VMR CV1 DO1YQ1MY1&1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
152 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - change in conduction velocity Vascular - BP lowering not characterized in autonomic section
-
REFERENCE :
-
JTCTDW Journal of Toxicology, Clinical Toxicology. (Marcel Dekker, 270 Madison Ave., New York, NY 10016) V.19- 1982- Volume(issue)/page/year: 20,69,1983
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
137 mg/kg/24D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting Kidney, Ureter, Bladder - other changes in urine composition Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 111,533,1989
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
109 mg/kg/22D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting Kidney, Ureter, Bladder - other changes in urine composition Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 111,533,1989
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
125 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CKFRAY Ceskoslovenska Farmacie. (PNS-Ustredni Expedice a Dovoz Tisku, Kafkova 19, 160 00 Prague 6, Czechoslovakia) V.1- 1952- Volume(issue)/page/year: 42,82,1993
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
75200 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NPMDAD Nouvelle Presse Medicale. (Paris, France) V.1-11, 1972-82. Volume(issue)/page/year: 7,3258,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
4 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - change in rate Vascular - BP lowering not characterized in autonomic section Kidney, Ureter, Bladder - changes in blood vessels or in circulation of kidney
-
REFERENCE :
-
MASODV Masui to Sosei. Anesthesia and Resuscitation. (Hiroshima Masui Igakkai, Hiroshima Daigaku Igakubu Masuigaku Kyoshitsu, 1-2-3 Kasumi, Minami-ku, Hiroshima, Hiroshima, Japan) V.6- 1970- Volume(issue)/page/year: 16,13,1980 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 34-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - respiratory system
-
REFERENCE :
-
DPTHDL Developmental Pharmacology and Therapeutics. (S. Karger Pub., Inc., 79 Fifth Ave., New York, NY 10003) V.1- 1980- Volume(issue)/page/year: 4(Suppl 1),109,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
1080 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - biochemical and metabolic
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 283,1077,1981
|